Abstract
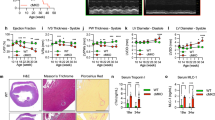
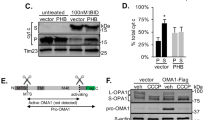
Mitochondria play an important role in energy production, Ca2+ homeostasis and cell death. In recent years, the role of the mitochondria in apoptotic and necrotic cell death has attracted much attention1,2. In apoptosis and necrosis, the mitochondrial permeability transition (mPT), which leads to disruption of the mitochondrial membranes and mitochondrial dysfunction, is considered to be one of the key events, although its exact role in cell death remains elusive. We therefore created mice lacking cyclophilin D (CypD), a protein considered to be involved in the mPT, to analyse its role in cell death. CypD-deficient mice were developmentally normal and showed no apparent anomalies, but CypD-deficient mitochondria did not undergo the cyclosporin A-sensitive mPT. CypD-deficient cells died normally in response to various apoptotic stimuli, but showed resistance to necrotic cell death induced by reactive oxygen species and Ca2+ overload. In addition, CypD-deficient mice showed a high level of resistance to ischaemia/reperfusion-induced cardiac injury. Our results indicate that the CypD-dependent mPT regulates some forms of necrotic death, but not apoptotic death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tsujimoto, Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J. Cell. Physiol. 195, 158–167 (2003)
Green, D. R. & Kroemer, G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004)
Halestrap, A. P., McStay, G. P. & Clarke, S. J. The permeability transition pore complex: another view. Biochimie 84, 153–166 (2002)
Crompton, M. On the involvement of mitochondrial intermembrane junctional complexes in apoptosis. Curr. Med. Chem. 10, 1473–1484 (2003)
Kokoszka, J. E. et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 (2004)
Halestrap, A. P. Mitochondrial permeability: dual role for the ADP/ATP translocator? Nature [online] 430, 983 (2004) (doi:10.1038/nature02816)
Galat, A. & Metcalfe, S. M. Peptidylproline cis/trans isomerases. Prog. Biophys. Mol. Biol. 63, 67–118 (1995)
Broekemeier, K. M., Dempsey, M. E. & Pfeiffer, D. R. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 264, 7826–7830 (1989)
He, L. & Lemasters, J. J. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 512, 1–7 (2002)
Scorrano, L. et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55–67 (2002)
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001)
Narita, M. et al. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl Acad. Sci. USA 95, 14681–14686 (1998)
Eskes, R. et al. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 143, 217–224 (1998)
Finucane, D. M., Bossy-Wetzel, E., Waterhouse, N. J., Cotter, T. G. & Green, D. R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274, 2225–2233 (1999)
von Ahsen, O. et al. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J. Cell Biol. 150, 1027–1036 (2000)
Trollinger, D. R., Cascio, W. E. & Lemasters, J. J. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem. Biophys. Res. Commun. 236, 738–742 (1997)
Weiss, J. N., Korge, P., Honda, H. M. & Ping, P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 93, 292–301 (2003)
Shimizu, S. et al. Beneficial effects of cyclosporine on reoxygenation injury in hypoxic rat liver. Transplantation 57, 1562–1566 (1994)
Javadov, S. A. et al. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J. Physiol. (Lond.) 549, 513–524 (2003)
Matsumoto, S., Friberg, H., Ferrand-Drake, M. & Wieloch, T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 19, 736–741 (1999)
Khaspekov, L., Friberg, H., Halestrap, A., Viktorov, I. & Wieloch, T. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur. J. Neurosci. 11, 3194–3198 (1999)
Zamzami, N. & Kroemer, G. The mitochondrion in apoptosis: how Pandora's box opens. Nature Rev. Mol. Cell Biol. 2, 67–71 (2001)
Li, Y., Johnson, N., Capano, M., Edwards, M. & Crompton, M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochem. J. 383, 101–109 (2004)
Shimizu, S. et al. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl Acad. Sci. USA 95, 1455–1459 (1998)
Fischer, G., Wittmann-Liebold, B., Lang, K., Kiefhaber, T. & Schmid, F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337, 476–478 (1989)
Hatano, E. et al. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J. Biol. Chem. 275, 11814–11823 (2000)
Shinzawa, K. & Tsujimoto, Y. PLA2 activity is required for nuclear shrinkage in caspase-independent cell death. J. Cell Biol. 163, 1219–1230 (2003)
Shimizu, S., Eguchi, Y., Kamiike, W., Matsuda, H. & Tsujimoto, Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene 12, 2251–2257 (1996)
Byrne, A. M., Lemasters, J. J. & Nieminen, A. L. Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology 29, 1523–1531 (1999)
Yamashita, N. et al. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J. Exp. Med. 189, 1699–1706 (1999)
Acknowledgements
We are grateful to K. Tagawa for helpful discussion and C. Thompson for providing Bak-deficient mice. CypD-deficient mice were developed in collaboration with Lexicon Genetics Incorporated. This study was supported in part by a grant for Scientific Research on Priority Areas, a grant for Center of Excellence Research, a grant for the 21st century COE Program, a grant for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan, and by a grant for Research on Dementia and Fracture from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Creation of Cyp D-deficient Mice. (PDF 2240 kb)
Supplementary Figure 2
No difference in respiration rate between control and Cyp D-deficient mitochondria. (PDF 1047 kb)
Supplementary Figure 3
High doses of Ca2+ cause ΔΨ loss in Cyp D-deficient mitochondria. (PDF 528 kb)
Supplementary Figure 4
Absence of mPT in Cyp D-deficient mitochondria by mPT inducers. (PDF 48 kb)
Supplementary Figure 5
Resistance of ΔΨ loss by Cyp D deficiency. (PDF 429 kb)
Supplementary Table
Analysis of cardiac sizes and functions by echocardiography. (DOC 25 kb)
Rights and permissions
About this article
Cite this article
Nakagawa, T., Shimizu, S., Watanabe, T. et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 (2005). https://doi.org/10.1038/nature03317
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03317
This article is cited by
-
Mitochondria in endothelial cells angiogenesis and function: current understanding and future perspectives
Journal of Translational Medicine (2023)
-
Lysosomal Ca2+ as a mediator of palmitate-induced lipotoxicity
Cell Death Discovery (2023)
-
Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions
Cell Death & Differentiation (2023)
-
Necrocide 1 mediates necrotic cell death and immunogenic response in human cancer cells
Cell Death & Disease (2023)
-
Alpha Particle–Emitting Radiopharmaceuticals as Cancer Therapy: Biological Basis, Current Status, and Future Outlook for Therapeutics Discovery
Molecular Imaging and Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.