Abstract
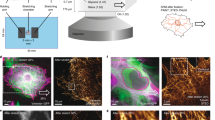
The mechanical environment crucially influences many cell functions1. However, it remains largely mysterious how mechanical stimuli are transmitted into biochemical signals. Src is known to regulate the integrin–cytoskeleton interaction2, which is essential for the transduction of mechanical stimuli3,4,5. Using fluorescent resonance energy transfer (FRET), here we develop a genetically encoded Src reporter that enables the imaging and quantification of spatio-temporal activation of Src in live cells. We introduced a local mechanical stimulation to human umbilical vein endothelial cells (HUVECs) by applying laser-tweezer traction on fibronectin-coated beads adhering to the cells. Using the Src reporter, we observed a rapid distal Src activation and a slower directional wave propagation of Src activation along the plasma membrane. This wave propagated away from the stimulation site with a speed (mean ± s.e.m.) of 18.1 ± 1.7 nm s-1. This force-induced directional and long-range activation of Src was abolished by the disruption of actin filaments or microtubules. Our reporter has thus made it possible to monitor mechanotransduction in live cells with spatio-temporal characterization. We find that the transmission of mechanically induced Src activation is a dynamic process that directs signals via the cytoskeleton to spatial destinations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ingber, D. E. Tensegrity. II. How structural networks influence cellular information processing networks. J. Cell. Sci. 116, 1397–1408 (2003)
Felsenfeld, D. P., Schwartzberg, P. L., Venegas, A., Tse, R. & Sheetz, M. P. Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nature Cell Biol. 1, 200–206 (1999)
Critchley, D. R. Focal adhesions—the cytoskeletal connection. Curr. Opin. Cell Biol. 12, 133–139 (2000)
Chicurel, M. E., Singer, R. H., Meyer, C. J. & Ingber, D. E. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature 392, 730–733 (1998)
Jiang, G., Giannone, G., Critchley, D. R., Fukumoto, E. & Sheetz, M. P. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424, 334–337 (2003)
Alenghat, F. J. & Ingber, D. E. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci. STKE 2002, E6 (2002)
Arias-Salgado, E. G. et al. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl Acad. Sci. USA 100, 13298–13302 (2003)
Fincham, V. J., Brunton, V. G. & Frame, M. C. The SH3 domain directs acto-myosin-dependent targeting of v-Src to focal adhesions via phosphatidylinositol 3-kinase. Mol. Cell Biol. 20, 6518–6536 (2000)
Kaplan, G. et al. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 15, 4282–4296 (1996)
Ting, A. Y., Kain, K. H., Klemke, R. L. & Tsien, R. Y. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc. Natl Acad. Sci. USA 98, 15003–15008 (2001)
Hamasaki, K. et al. Src kinase plays an essential role in integrin-mediated tyrosine phosphorylation of Crk-associated substrate p130Cas. Biochem. Biophys. Res. Commun. 222, 338–343 (1996)
Vuori, K., Hirai, H., Aizawa, S. & Ruoslahti, E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol. Cell Biol. 16, 2606–2613 (1996)
Songyang, Z. et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 373, 536–539 (1995)
Yang, F., Liu, Y., Bixby, S. D., Friedman, J. D. & Shokat, K. M. Highly efficient green fluorescent protein-based kinase substrates. Anal. Biochem. 266, 167–173 (1999)
Waksman, G. et al. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature 358, 646–653 (1992)
Bradshaw, J. M. & Waksman, G. Calorimetric examination of high-affinity Src SH2 domain-tyrosyl phosphopeptide binding: dissection of the phosphopeptide sequence specificity and coupling energetics. Biochemistry 38, 5147–5154 (1999)
Yang, F., Moss, L. G. & Phillips, G. N. Jr The molecular structure of green fluorescent protein. Nature Biotechnol. 14, 1246–1251 (1996)
Zacharias, D. A., Violin, J. D., Newton, A. C. & Tsien, R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002)
Miyamoto, S. et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131, 791–805 (1995)
Tsien, R. Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998)
Thomas, S. M. & Brugge, J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 (1997)
Heidermann, S. R., Kaech, S., Buxbaum, R. E. & Matus, A. Direct observations of the mechanical behaviors of the cytoskeleton in living fibroblasts. J. Cell Biol. 145, 109–122 (1999)
Hu, S. et al. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Physiol Cell Physiol. 285, C1082–C1090 (2003)
Mack, P. J., Kaazempur-Mofrad, M. R., Karcher, H., Lee, R. T. & Kamm, R. D. Force-induced focal adhesion translocation: effects of force amplitude and frequency. Am. J. Physiol. Cell Physiol. 287, C954–C962 (2004)
Riveline, D. et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 (2001)
Brugnera, E. et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nature Cell Biol. 4, 574–582 (2002)
Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998)
Sandilands, E. et al. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev. Cell 7, 855–869 (2004)
Timpson, P., Jones, G. E., Frame, M. C. & Brunton, V. G. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 11, 1836–1846 (2001)
Otsu, N. Threshold selection method from Gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979)
Acknowledgements
We thank J. Zhang and A. Y. Ting for their assistance and advice; J.-L. Guan, D. C. Flynn and J. Cooper for providing FAK protein, MEF cells, c-Yes plasmid and SYF cell lines. We also thank B. N. G. Giepmans and C. Hauser for their comments on the paper, and Q. Xiong for technical support. This work was supported by the National Institutes of Health (S.C., R.Y.T. and M.W.B.), the Howard Hughes Medical Institute (R.Y.T.), the Airforce Office of Scientific Research (M.W.B.), the Arnold and Mabel Beckman Foundation (E.L.B.) and the Alliance for Cellular Signaling Grant (R.Y.T., S.C. and Y.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Materials
This file contains Supplementary Methods, Supplementary Figure Legends and Supplementary Movie Legends. (DOC 72 kb)
Supplementary Figures
This file contains Supplementary Figures S1-S7. (PPT 1203 kb)
Supplementary Movie S1
The EGF-induced FRET response in HeLa cells expressing the Src reporter. (MOV 207 kb)
Supplementary Movie S2
The effects of EGF stimulation and washout on FRET responses of the monomeric Src reporter in HeLa cells. (MOV 4949 kb)
Supplementary Movie S3
A fibronectin-coated bead induced local FRET responses in HUVECs. (MOV 3253 kb)
Supplementary Movie S4
PP1 reversed the local FRET response in HUVECs induced by fibronectin-coated beads. (MOV 2471 kb)
Supplementary Movie S5
The laser-tweezer-traction on a fibronectin-coated bead induced FRET responses of the cytosolic Src reporter in HUVECs. (MOV 4093 kb)
Supplementary Movie S6
The laser-tweezer-traction on a polylysine-coated bead did not induce FRET responses in HUVECs. (MOV 8073 kb)
Supplementary Movie S7
PP1 reversed the EGF-induced FRET responses of the membrane-targeted Src reporter in HeLa cells. (MOV 459 kb)
Supplementary Movie S8
Pretreatment with PP1 inhibited the EGF-induced FRET responses of the membrane-targeted Src reporter in HeLa cells. (MOV 1017 kb)
Supplementary Movie S9
The laser-tweezer-traction induced directional and long-range propagation of FRET responses of the membrane-targeted Src reporter in HUVECs. (MOV 6434 kb)
Supplementary Movie S10
The laser-tweezer traction on a fibronectin-coated bead did not induce FRET responses of the Y662F/Y664F mutant of the membrane-targeted Src reporter. (MOV 1759 kb)
Supplementary Movie S11
The laser-tweezer traction on a fibronectin-coated bead did not induce FRET responses of the R175V mutant of the membrane-targeted Src reporter. (MOV 1684 kb)
Supplementary Movie S12
A highlighted cell with a clear FRET wave propagation away from the stimulation site upon force application. (MOV 7469 kb)
Supplementary Movie S13
Cytochalasin D blocked the directional and long-range activation of Src in response to mechanical force. (MOV 6768 kb)
Supplementary Movie S14
Nocodazole blocked the directional and long-range activation of Src in response to mechanical force. (MOV 8909 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Botvinick, E., Zhao, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005). https://doi.org/10.1038/nature03469
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03469
This article is cited by
-
Acute Noise Causes Down-Regulation of ECM Protein Expression in Guinea Pig Cochlea
Molecular Biotechnology (2023)
-
Matrix stiffness, endothelial dysfunction and atherosclerosis
Molecular Biology Reports (2023)
-
CdGAP maintains podocyte function and modulates focal adhesions in a Src kinase-dependent manner
Scientific Reports (2022)
-
Intracellular detection and communication of a wireless chip in cell
Scientific Reports (2021)
-
Forces generated by lamellipodial actin filament elongation regulate the WAVE complex during cell migration
Nature Cell Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.