Abstract
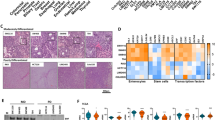
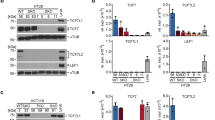
Most sporadic colorectal cancers are initiated by activating Wnt pathway mutations1, characterized by the stabilization of β-catenin and constitutive transcription by the β-catenin/T cell factor-4 (Tcf-4) complex2,3. EphB guidance receptors are Tcf4 target genes that control intestinal epithelial architecture through repulsive interactions with Ephrin-B ligands4,5. Here we show that, although Wnt signalling remains constitutively active, most human colorectal cancers lose expression of EphB at the adenoma–carcinoma transition. Loss of EphB expression strongly correlates with degree of malignancy. Furthermore, reduction of EphB activity accelerates tumorigenesis in the colon and rectum of ApcMin/+ mice, and results in the formation of aggressive adenocarcinomas. Our data demonstrate that loss of EphB expression represents a critical step in colorectal cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bienz, M. & Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 103, 311–320 (2000)
Korinek, V. et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275, 1784–1787 (1997)
Morin, P. J. et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275, 1787–1790 (1997)
Batlle, E. et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111, 251–263 (2002)
van de Wetering, M. et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002)
Kuhnert, F. et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl Acad. Sci. USA 101, 266–271 (2004)
Powell, S. M. et al. APC mutations occur early during colorectal tumorigenesis. Nature 359, 235–237 (1992)
Sparks, A. B., Morin, P. J., Vogelstein, B. & Kinzler, K. W. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 58, 1130–1134 (1998)
Shitoh, K. et al. Frequent activation of the beta-catenin-Tcf signaling pathway in nonfamilial colorectal carcinomas with microsatellite instability. Genes Chromosom. Cancer 30, 32–37 (2001)
Su, L. K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992)
Moser, A. R., Pitot, H. C. & Dove, W. F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990)
Yamada, Y. et al. Microadenomatous lesions involving loss of Apc heterozygosity in the colon of adult Apc(Min/ + ) mice. Cancer Res. 62, 6367–6370 (2002)
Huusko, P. et al. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nature Genet. 36, 979–983 (2004)
Sancho, E., Batlle, E. & Clevers, H. Live and let die in the intestinal epithelium. Curr. Opin. Cell Biol. 15, 763–770 (2003)
Mao, W. et al. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 64, 781–788 (2004)
Munarini, N. et al. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J. Cell Sci. 115, 25–37 (2002)
Gregorieff, A., Grosschedl, R. & Clevers, H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(- / - )/Tcf1(- / - ) embryos. EMBO J. 23, 1825–1833 (2004)
Orioli, D., Henkemeyer, M., Lemke, G., Klein, R. & Pawson, T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 15, 6035–6049 (1996)
Roose, J. et al. Synergy between tumour suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285, 1923–1926 (1999)
Lal, G. et al. Suppression of intestinal polyps in Msh2-deficient and non-Msh2-deficient multiple intestinal neoplasia mice by a specific cyclooxygenase-2 inhibitor and by a dual cyclooxygenase-1/2 inhibitor. Cancer Res. 61, 6131–6136 (2001)
Acknowledgements
We thank A. Ziemiecki for EphB4 antiserum, and A. García de Herreros and C. Francí for help with mouse experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
Detailed immunohistochemistry and in situ hybridisation protocols. (PDF 20 kb)
Supplementary Figure Legends
Legends for Supplementary Figures S1-S11. (PDF 74 kb)
Supplementary Figure S1
Example of loss of EphB2 expression in CRC liver metastasis. (PDF 206 kb)
Supplementary Figure S2
Examples of colorectal lesions classified in each EphB group. (PDF 306 kb)
Supplementary Figure S3
Statistical analysis of EphB2 expression in CRC lesions. (PDF 31 kb)
Supplementary Figure S4
Statistical analysis of EphB4 expression in CRC lesions. (PDF 30 kb)
Supplementary Figure S5
Statistical analysis of the correlation between EphB2 and EphB4 expression in CRC lesions. (PDF 11 kb)
Supplementary Figure S6
Example of colorectal tumour with downregulated EphB2 protein but high levels of EphB2 mRNA. (PDF 243 kb)
Supplementary Figure S7
Examples of in situ hybridisation with EphB2 and EphB3 probes on carcinomas with different histological grade. (PDF 149 kb)
Supplementary Figure S8
EphB4 expression in intestine of EphB2 and -B3 knock-out mice. EphB4 mRNA levels in CRC cell lines upon induction of dominant negative TCF factors. (PDF 177 kb)
Supplementary Figure 9
Examples of EphB4 expression patterns in CRC lesions at different stages. (PDF 313 kb)
Supplementary Figure S10
Nuclear β-catenin accumulation and expression of EphB2 and EphB4 in colonic microlesions of APCmin/+ mice. (PDF 205 kb)
Supplementary Figure S11
Features of δcyEphB2;ApcMin/+ tumours including nuclear β-catenin accumulation. (PDF 197 kb)
Rights and permissions
About this article
Cite this article
Batlle, E., Bacani, J., Begthel, H. et al. EphB receptor activity suppresses colorectal cancer progression. Nature 435, 1126–1130 (2005). https://doi.org/10.1038/nature03626
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03626
This article is cited by
-
Eph receptors and ephrins in cancer progression
Nature Reviews Cancer (2024)
-
Ephrin–Eph receptor tyrosine kinases for potential therapeutics against hepatic pathologies
Journal of Cell Communication and Signaling (2023)
-
Long non-coding RNAs as the critical regulators of epithelial mesenchymal transition in colorectal tumor cells: an overview
Cancer Cell International (2022)
-
TAGAP instructs Th17 differentiation by bridging Dectin activation to EPHB2 signaling in innate antifungal response
Nature Communications (2020)
-
Receptor Tyrosine Kinase EphB3: a Prognostic Indicator in Colorectal Carcinoma
Pathology & Oncology Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.