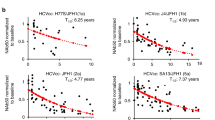
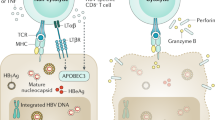
Abstract
The hepatitis C virus (HCV) persists in the majority of infected individuals and is a significant cause of human illness and death globally. Recent studies have yielded important insights into immunity to HCV, in particular revealing the central role of T cells in viral control and clearance. Other key features of adaptive immune responses remain obscure, including mechanisms by which T cells control HCV replication, the role of antibodies in conferring protection and how cellular and humoral immunity are subverted in persistent infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bowmen, D. G. & Walker, C. M. The origin of quasispecies: cause or consequence of chronic hepatitis C viral infection? J. Hepatol. 42, 408–417 (2005).
Pearlman, B. L. Hepatitis C treatment update. Am. J. Med. 117, 344–352 (2004).
Shoukry, N. H., Cawthon, A. G. & Walker, C. M. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu. Rev. Microbiol. 58, 391–424 (2004).
Pawlotsky, J. M. Diagnostic tests for hepatitis C. J. Hepatol. 31 (Suppl 1), 71–79 (1999).
Farci, P. et al. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc. Natl Acad. Sci. USA 91, 7792–7796 (1994).
Farci, P. et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288, 339–344 (2000).
Farci, P. et al. Lack of protective immunity against reinfection with hepatitis C virus. Science 258, 135–140 (1992).
Lai, M. E. et al. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet 343, 388–390 (1994).
Cooper, S. et al. Analysis of a successful immune response against hepatitis C virus. Immunity 10, 439–449 (1999).
Post, J. J. et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J. Infect. Dis. 189, 1846–1855 (2004).
Bartosch, B. et al. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl Acad. Sci. USA 100, 14199–14204 (2003).
Logvinoff, C. et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc. Natl Acad. Sci. USA 101, 10149–10154 (2004).
Meunier, J. C. et al. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc. Natl Acad. Sci. USA 102, 4560–4565 (2005).
Negro, F. et al. Detection of intrahepatic replication of hepatitis C virus RNA by in situ hybridization and comparison with histopathology. Proc. Natl Acad. Sci. USA 89, 2247–2251 (1992).
Nouri-Aria, K. T., Sallie, R., Mizokami, M., Portmann, B. C. & Williams, R. Intrahepatic expression of hepatitis C virus antigens in chronic liver disease. J. Pathol. 175, 77–83 (1995).
Abe, K., Inchauspe, G., Shikata, T. & Prince, A. M. Three different patterns of hepatitis C virus infection in chimpanzees. Hepatology 15, 690–695 (1992).
Beach, M. J. et al. Temporal relationships of hepatitis C virus RNA and antibody responses following experimental infection of chimpanzees. J. Med. Virol. 36, 226–237 (1992).
Alter, H. J. et al. Evaluation of branched DNA signal amplification for the detection of hepatitis C virus RNA. J. Viral Hepatol. 2, 121–132 (1995).
Missale, G. et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Invest. 98, 706–714 (1996).
Diepolder, H. M. et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346, 1006–1007 (1995).
Gerlach, J. T. et al. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology 117, 933–941 (1999).
Thimme, R. et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194, 1395–1406 (2001).
Thimme, R. et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl Acad. Sci. USA 99, 15661–15668 (2002).
Shoukry, N. H. et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197, 1645–1655 (2003).
Lechner, F. et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30, 2479–2487 (2000).
Frese, M. et al. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35, 694–703 (2002).
Lanford, R. E. et al. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(i)-poly(c), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77, 1092–1104 (2003).
Rehermann, B. et al. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J. Clin. Invest. 98, 1432–1440 (1996).
Nelson, D. R. et al. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J. Immunol. 158, 1473–1481 (1997).
Wong, D. K. et al. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J. Immunol. 160, 1479–1488 (1998).
Freeman, A. J. et al. The presence of an intrahepatic cytotoxic T lymphocyte response is associated with low viral load in patients with chronic hepatitis C virus infection. J. Hepatol. 38, 349–356 (2003).
He, X. S. et al. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl Acad. Sci. USA 96, 5692–5697 (1999).
Kurokohchi, K. et al. CD28-negative CD8-positive cytotoxic T lymphocytes mediate hepatocellular damage in hepatitis C virus infection. J. Clin. Immunol. 23, 518–527 (2003).
Abrignani, S. Bystander activation by cytokines of intrahepatic T cells in chronic viral hepatitis. Semin. Liver Dis. 17, 319–322 (1997).
Napoli, J., Bishop, G. A., McGuinness, P. H., Painter, D. M. & McCaughan, G. W. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 24, 759–765 (1996).
McGuinness, P. H., Painter, D., Davies, S. & McCaughan, G. W. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut 46, 260–269 (2000).
Lechner, F. et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191, 1499–1512 (2000).
Grakoui, A. et al. HCV persistence and immune evasion in the absence of memory T cell help. Science 302, 659–662 (2003).
Koziel, M. J. et al. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J. Virol. 67, 7522–7532 (1993).
Takaki, A. et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nature Med. 6, 578–582 (2000).
Wedemeyer, H. et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169, 3447–3458 (2002).
Lauer, G. M. et al. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127, 924–936 (2004).
Urbani, S. et al. Heterologous T cell immunity in severe hepatitis C virus infection. J. Exp. Med. 201, 675–680 (2005).
Koziel, M. J. et al. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J. Immunol. 149, 3339–3344 (1992).
Erickson, A. L. et al. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15, 883–895 (2001).
Kimura, Y., Gushima, T., Rawale, S., Kaumaya, P. & Walker, C. M. Escape mutations alter proteasome processing of major histocompatibility complex class I-restricted epitopes in persistent hepatitis C virus infection. J. Virol. 79, 4870–4876 (2005).
Meyer-Olson, D. et al. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J. Exp. Med. 200, 307–319 (2004).
Day, C. L. et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J. Virol. 76, 12584–12595 (2002).
Shoukry, N. H., Sidney, J., Sette, A. & Walker, C. M. Conserved hierarchy of helper T cell responses in a chimpanzee during primary and secondary hepatitis C virus infections. J. Immunol. 172, 483–492 (2004).
Mehta, S. H. et al. Protection against persistence of hepatitis C. Lancet 359, 1478–1483 (2002).
Bassett, S. E. et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33, 1479–1487 (2001).
Major, M. E. et al. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol. 76, 6586–6595 (2002).
Hahn, Y. S. Subversion of immune responses by hepatitis C virus: immunomodulatory strategies beyond evasion? Curr. Opin. Immunol. 15, 443–449 (2003).
Accapezzato, D. et al. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: the role of the virus. Eur. J. Immunol. 34, 438–446 (2004).
Khakoo, S. I. et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004).
Crotta, S. et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195, 35–41 (2002).
Tseng, C. T. & Klimpel, G. R. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195, 43–49 (2002).
Jinushi, M. et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J. Immunol. 173, 6072–6081 (2004).
Kanto, T. et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 162, 5584–5591 (1999).
Auffermann-Gretzinger, S., Keeffe, E. B. & Levy, S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97, 3171–3176 (2001).
Bain, C. et al. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120, 512–524 (2001).
Larsson, M. et al. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J. Virol. 78, 6151–6161 (2004).
Piccioli, D. et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J. Hepatol. 42, 61–67 (2005).
Bukh, J., Miller, R. H. & Purcell, R. H. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin. Liver Dis. 15, 41–63 (1995).
Bowen, D. G. & Walker, C. M. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med. 201, 1709–1714 (2005).
Chang, K. M. et al. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J. Clin. Invest. 100, 2376–2385 (1997).
Timm, J. et al. CD8 epitope escape and reversion in acute HCV infection. J. Exp. Med. 200, 1593–1604 (2004).
Tester, I. et al. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J. Exp. Med. 201, 1725–1731 (2005).
Cox, A. L. et al. Cellular immune selection with hepatitis C virus persistence in humans. J. Exp. Med. 201, 1741–1752 (2005).
Ray, S. C. et al. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J. Exp. Med. 201, 1753–1759 (2005).
Leslie, A. J. et al. HIV evolution: CTL escape mutation and reversion after transmission. Nature Med. 10, 282–289 (2004).
Friedrich, T. C. et al. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nature Med. 10, 275–281 (2004).
Leslie, A. et al. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J. Exp. Med. 201, 891–902 (2005).
Moore, C. B. et al. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science 296, 1439–1443 (2002).
Altfeld, M. et al. The majority of currently circulating human immunodeficiency virus type 1 clade B viruses fail to prime cytotoxic T-lymphocyte responses against an otherwise immunodominant HLA-A2-restricted epitope: implications for vaccine design. J. Virol. 79, 5000–5005 (2005).
Lauer, G. M. et al. Comprehensive analysis of CD8+-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J. Virol. 76, 6104–6113 (2002).
Wang, J. H., Layden, T. J. & Eckels, D. D. Modulation of the peripheral T-cell response by CD4 mutants of hepatitis C virus: transition from a Th1 to a Th2 response. Hum. Immunol. 64, 662–673 (2003).
Frasca, L. et al. Hypervariable region 1 variants act as TCR antagonists for hepatitis C virus-specific CD4+ T cells. J. Immunol. 163, 650–658 (1999).
Wang, H. & Eckels, D. D. Mutations in immunodominant T cell epitopes derived from the nonstructural 3 protein of hepatitis C virus have the potential for generating escape variants that may have important consequences for T cell recognition. J. Immunol. 162, 4177–4183 (1999).
Penna, A. et al. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: functional characterization in patients with chronic hepatitis C. Hepatology 35, 1225–1236 (2002).
Moskophidis, D., Lechner, F., Pircher, H. & Zinkernagel, R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993).
Gruener, N. H. et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75, 5550–5558 (2001).
Urbani, S. et al. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76, 12423–12434 (2002).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8, 379–385 (2002).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Khanolkar, A., Fuller, M. J. & Zajac, A. J. CD4 T cell-dependent CD8 T cell maturation. J. Immunol. 172, 2834–2844 (2004).
Lucas, M. et al. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J. Immunol. 172, 1744–1753 (2004).
Rosen, H. R. et al. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology 35, 190–198 (2002).
Day, C. L. et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Invest. 112, 831–842 (2003).
Ulsenheimer, A. et al. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology 37, 1189–1198 (2003).
MacDonald, A. J. et al. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J. Infect. Dis. 185, 720–727 (2002).
Semmo, N. et al. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology 41, 1019–1028 (2005).
Koziel, M. J. et al. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Invest. 96, 2311–2321 (1995).
Accapezzato, D. et al. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J. Clin. Invest. 113, 963–972 (2004).
Sugimoto, K. et al. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38, 1437–1448 (2003).
Cabrera, R. et al. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 40, 1062–1071 (2004).
Boettler, T. et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J. Virol. 79, 7860–7867 (2005).
Rushbrook, S. M. et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J. Virol. 79, 7852–7859 (2005).
Bertolino, P., McCaughan, G. W. & Bowen, D. G. Role of primary intrahepatic T-cell activation in the ‘liver tolerance effect’. Immunol. Cell Biol. 80, 84–92 (2002).
Lanford, R. E. et al. Cross-genotype immunity to hepatitis C virus. J. Virol. 78, 1575–1581 (2004).
Acknowledgements
Our research was supported by Public Health Service grants to C.M.W. D.G.B. was supported by a C.J. Martin Fellowship from the National Health and Medical Research Council of Australia and an AstraZeneca Fellowship in Medical Research from the Royal Australasian College of Physicians.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Rights and permissions
About this article
Cite this article
Bowen, D., Walker, C. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436, 946–952 (2005). https://doi.org/10.1038/nature04079
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04079
This article is cited by
-
A hepatic network of dendritic cells mediates CD4 T cell help outside lymphoid organs
Nature Communications (2024)
-
Body mass index (BMI) and alpha-fetoprotein (AFP) level correlate with the severity of HCV-induced fibrosis in a cohort of Egyptian patients with chronic HCV
Future Journal of Pharmaceutical Sciences (2020)
-
The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity
Cell Research (2020)
-
Targeting p53 and histone methyltransferases restores exhausted CD8+ T cells in HCV infection
Nature Communications (2020)
-
Exploring the interactions between the human and viral genomes
Human Genetics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.