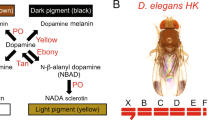
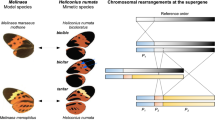
Abstract
The independent evolution of morphological similarities is widespread1,2. For simple traits, such as overall body colour, repeated transitions by means of mutations in the same gene may be common3,4,5. However, for more complex traits, the possible genetic paths may be more numerous; the molecular mechanisms underlying their independent origins and the extent to which they are constrained to follow certain genetic paths are largely unknown. Here we show that a male wing pigmentation pattern involved in courtship display has been gained and lost multiple times in a Drosophila clade. Each of the cases we have analysed (two gains and two losses) involved regulatory changes at the pleiotropic pigmentation gene yellow. Losses involved the parallel inactivation of the same cis-regulatory element (CRE), with changes at a few nucleotides sufficient to account for the functional divergence of one element between two sibling species. Surprisingly, two independent gains of wing spots resulted from the co-option of distinct ancestral CREs. These results demonstrate how the functional diversification of the modular CREs of pleiotropic genes contributes to evolutionary novelty and the independent evolution of morphological similarities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Conway Morris, S. Life's Solution. Inevitable Humans in a Lonely Universe (Cambridge Univ. Press, Cambridge, 2005)
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford Univ. Press, Oxford, 2003)
Protas, M. E. et al. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature Genet. 38, 107–111 (2006)
Nachman, M. W., Hoekstra, H. E. & D'Agostino, S. L. The genetic basis of adaptive melanism in pocket mice. Proc. Natl Acad. Sci. USA 100, 5268–5273 (2003)
Theron, E., Hawkins, K., Bermingham, E., Ricklefs, R. E. & Mundy, N. I. The molecular basis of an avian plumage polymorphism in the wild: a melanocortin-1-receptor point mutation is perfectly associated with the melanic plumage morph of the bananaquit, Coereba flaveola. Curr. Biol. 11, 550–557 (2001)
Bock, I. R. & Wheeler, M. R. in Studies in Genetics (ed. Wheeler, M. R.) 1–102 (University of Texas, Austin, 1972)
Lakovaara, S. & Saura, A. in The Genetics and Biology of Drosophila Vol. 3b (eds Ashburner, M., Carson, H. L. & Thompson, J. N.) 1–59 (Academic, London, 1982)
Gompel, N., Prud'homme, B., Wittkopp, P. J., Kassner, V. A. & Carroll, S. B. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433, 481–487 (2005)
Pagel, M., Meade, A. & Barker, D. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 53, 673–684 (2004)
Huelsenbeck, J. P., Rannala, B. & Masly, J. P. Accommodating phylogenetic uncertainty in evolutionary studies. Science 288, 2349–2350 (2000)
Ronquist, F. Bayesian inference of character evolution. Trends Ecol. Evol. 19, 475–481 (2004)
Yeh, S.-D., Liou, S.-R. & True, J. R. Genetics of divergence in male wing pigmentation and courtship behavior between Drosophila elegans and D. gunungcola. Heredity (in the press)
Wittkopp, P. J., Vaccaro, K. & Carroll, S. B. Evolution of yellow gene regulation and pigmentation in Drosophila. Curr. Biol. 12, 1547–1556 (2002)
Wittkopp, P. J., True, J. R. & Carroll, S. B. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 129, 1849–1858 (2002)
Jacob, F. Evolution and tinkering. Science 196, 1161–1166 (1977)
Drapeau, M. D., Cyran, S. A., Viering, M. M., Geyer, P. K. & Long, A. D. A cis-regulatory sequence within the yellow locus of Drosophila melanogaster required for normal male mating success. Genetics 172, 1009–1030 (2006)
Walter, M. F. et al. Temporal and spatial expression of the yellow gene in correlation with cuticle formation and dopa decarboxylase activity in Drosophila development. Dev. Biol. 147, 32–45 (1991)
Shapiro, M. D. et al. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature 428, 717–723 (2004)
Sucena, E., Delon, I., Jones, I., Payre, F. & Stern, D. L. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature 424, 935–938 (2003)
Sucena, E. & Stern, D. L. Divergence of larval morphology between Drosophila sechellia and its sibling species caused by cis-regulatory evolution of ovo/shaven-baby. Proc. Natl Acad. Sci. USA 97, 4530–4534 (2000)
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001)
Swofford, D. L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) 4.0 Beta (Sinauer Associates, Sunderland, Massachusetts, 2002)
Guindon, S. & Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 (2003)
O'Grady, P. M. & Kidwell, M. G. Phylogeny of the subgenus Sophophora (Diptera: Drosophilidae) based on combined analysis of nuclear and mitochondrial sequences. Mol. Phylogenet. Evol. 22, 442–453 (2002)
O'Grady, P. M. Reevaluation of phylogeny in the Drosophila obscura species group based on combined analysis of nucleotide sequences. Mol. Phylogenet. Evol. 12, 124–139 (1999)
Goto, S. G. & Kimura, M. T. Phylogenetic utility of mitochondrial COI and nuclear Gpdh genes in Drosophila. Mol. Phylogenet. Evol. 18, 404–422 (2001)
Kopp, A. & True, J. R. Phylogeny of the oriental Drosophila melanogaster species group: a multilocus reconstruction. Syst. Biol. 51, 786–805 (2002)
Schawaroch, V. Phylogeny of a paradigm lineage: the Drosophila melanogaster species group (Diptera: Drosophilidae). Biol. J. Linn. Soc. 76, 21–37 (2002)
Sanderson, M. J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003)
Acknowledgements
We thank A. Kopp for sharing unpublished data; J. J. Gao, S. Hagemann, M. T. Kimura, A. Kopp, V. Stamataki and the Drosophila Tucson Stock Center for providing fly stocks; M. Toda for fly identification; M. Bate for help with behavioural observations; A. Meade and M. Pagel for advice on bayesian ancestral character reconstruction; and B. Williams and J. Yoder for comments on the manuscript. N.G. thanks P. Simpson for hosting him in her laboratory. N.G. was an EMBO and a Human Frontier long-term fellow. The project was supported by the Howard Hughes Medical Institute (S.B.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The sequences described in this paper have been deposited at the EMBL nucleotide database under accession numbers AM181668 to AM181680. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Maximum Likelihood tree showing Bayesian Inference/Maximum Parsimony/Maximum Likelihood support value at each node. The tree includes 77 species of the melanogaster and obscura groups and two outgroup species from the willistoni group. (JPG 189 kb)
Supplementary Figure 2
D. mimetica (a) has lost its male wing spot independently from D. gunungcola, Yellow is not expressed at high levels in the putative spot region (b), as reflected by the uniform reporter expression across the wing of a wing largemim transformant (c). Specifically, the loss of Yellow expression in the prospective spot region is due to a non-functional spotmim element (d). (JPG 103 kb)
Supplementary Figure 3
Regulatory activity in the yellow intron and the origin of a spot. D. guanche adult males (a) are devoid of wing dark spot. However, the y intron from this species drives expression in the wing veins (b), as does the D. tristis y intron. In contrast, the y intron of species from the melanogaster group, such as D. biarmipes (c) does not drive spot or wing vein expression, but it does drive expression in the marginal sensory bristles of the wing. (JPG 77 kb)
Supplementary Movie 1
A sequence of courtship behaviour in Drosophila biarmipes. (MOV 4284 kb)
Supplementary Movie 2
A sequence of courtship behaviour in Drosophila tristis. (MOV 11451 kb)
Supplementary Method
Detail of the phylogenetic analysis. (DOC 75 kb)
Supplementary Table 1
Density of taxon sampling. (DOC 29 kb)
Supplementary Table 2
Number and percentage of loci and nucleotide sites per species in the data matrix. (DOC 58 kb)
Supplementary Table 3
List of primers. (DOC 52 kb)
Rights and permissions
About this article
Cite this article
Prud'homme, B., Gompel, N., Rokas, A. et al. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440, 1050–1053 (2006). https://doi.org/10.1038/nature04597
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04597
This article is cited by
-
The function and evolution of a genetic switch controlling sexually dimorphic eye differentiation in honeybees
Nature Communications (2023)
-
Distinct genetic architectures underlie divergent thorax, leg, and wing pigmentation between Drosophila elegans and D. gunungcola
Heredity (2021)
-
Functional traits for ecological studies: a review of characteristics of Drosophilidae (Diptera)
Community Ecology (2021)
-
Inverse resource allocation between vision and olfaction across the genus Drosophila
Nature Communications (2019)
-
The genetic architecture of adaptation: convergence and pleiotropy in Heliconius wing pattern evolution
Heredity (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.