Abstract
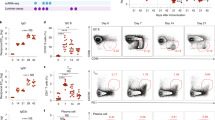
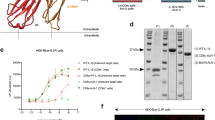
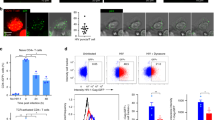
Successful vaccines contain not only protective antigen(s) but also an adjuvant component that triggers innate immune activation and is necessary for their optimal immunogenicity1,2. In the case of DNA vaccines3, this consists of plasmid DNA; however, the adjuvant element(s) as well as its intra- and inter-cellular innate immune signalling pathway(s) leading to the encoded antigen-specific T- and B-cell responses remain unclear. Here we demonstrate in vivo that TANK-binding kinase 1 (TBK1), a non-canonical IκB kinase, mediates the adjuvant effect of DNA vaccines and is essential for its immunogenicity in mice. Plasmid-DNA-activated, TBK1-dependent signalling and the resultant type-I interferon receptor-mediated signalling was required for induction of antigen-specific B and T cells, which occurred even in the absence of innate immune signalling through a well known CpG DNA sensor—Toll-like receptor 9 (TLR9) or Z-DNA binding protein 1 (ZBP1, also known as DAI, which was recently reported as a potential B-form DNA sensor4). Moreover, bone-marrow-transfer experiments revealed that TBK1-mediated signalling in haematopoietic cells was critical for the induction of antigen-specific B and CD4+ T cells, whereas in non-haematopoietic cells TBK1 was required for CD8+ T-cell induction. These data suggest that TBK1 is a key signalling molecule for DNA-vaccine-induced immunogenicity, by differentially controlling DNA-activated innate immune signalling through haematopoietic and non-haematopoietic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007)
Pulendran, B. & Ahmed, R. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124, 849–863 (2006)
Donnelly, J. J., Ulmer, J. B., Shiver, J. W. & Liu, M. A. DNA vaccines. Annu. Rev. Immunol. 15, 617–648 (1997)
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007)
Mata-Haro, V. et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316, 1628–1632 (2007)
Krieg, A. M. Therapeutic potential of Toll-like receptor 9 activation. Nature Rev. Drug Discov. 5, 471–484 (2006)
Gavin, A. L. et al. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314, 1936–1938 (2006)
Janssen, E. et al. Efficient T cell activation via a Toll–interleukin 1 receptor-independent pathway. Immunity 24, 787–799 (2006)
Yang, Z. Y. et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 428, 561–564 (2004)
Wang, R. et al. Induction of CD4+ T cell-dependent CD8+ type 1 responses in humans by a malaria DNA vaccine. Proc. Natl Acad. Sci. USA 98, 10817–10822 (2001)
Gurunathan, S., Klinman, D. M. & Seder, R. A. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18, 927–974 (2000)
Spies, B. et al. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J. Immunol. 171, 5908–5912 (2003)
Babiuk, S. et al. TLR9-/- and TLR9+/+ mice display similar immune responses to a DNA vaccine. Immunology 113, 114–120 (2004)
Tudor, D. et al. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine 23, 1258–1264 (2005)
Ulmer, J. B., Wahren, B. & Liu, M. A. Gene-based vaccines: recent technical and clinical advances. Trends Mol. Med. 12, 216–222 (2006)
Widera, G. et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo . J. Immunol. 164, 4635–4640 (2000)
Takeshita, F. et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J. Virol. 80, 6218–6224 (2006)
Ishii, K. J. & Akira, S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 27, 525–532 (2006)
Okabe, Y., Kawane, K., Akira, S., Taniguchi, T. & Nagata, S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 202, 1333–1339 (2005)
Ishii, K. J. et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nature Immunol. 7, 40–48 (2006)
Stetson, D. B. & Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24, 93–103 (2006)
Le Bon, A. & Tough, D. F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14, 432–436 (2002)
Baccala, R., Hoebe, K., Kono, D. H., Beutler, B. & Theofilopoulos, A. N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nature Med. 13, 543–551 (2007)
Hemmi, H. et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199, 1641–1650 (2004)
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000)
Condon, C., Watkins, S. C., Celluzzi, C. M., Thompson, K. & Falo, L. D. DNA-based immunization by in vivo transfection of dendritic cells. Nature Med. 2, 1122–1128 (1996)
Sasaki, S., Amara, R. R., Yeow, W. S., Pitha, P. M. & Robinson, H. L. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J. Virol. 76, 6652–6659 (2002)
Koyama, S. et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179, 4711–4720 (2007)
Ishii, K. J. et al. CpG-activated Thy1.2+ dendritic cells protect against lethal Listeria monocytogenes infection. Eur. J. Immunol. 35, 2397–2405 (2005)
Kaisho, T. et al. IκB kinase α is essential for mature B cell development and function. J. Exp. Med. 193, 417–426 (2001)
Acknowledgements
The authors thank T. Horii, K. Suzuki and S. Sasaki for suggestions, and Y. Fujita for technical support. This study was supported by Grant-in-Aid for Scientific Research (B) (to K.J.I.) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Author Contributions K.J.I., C.C. and S.A. designed the research and analysed data. K.J.I., S.K. and C.C. performed most experiments. T.K. generated ZBP-1-deficient mice and performed the related experiments. K.M. and O.T. performed the bone-marrow-transfer experiments. S.U., T.K. and H.K. provided mutant mice. F.T. provided critical materials and advice. K.J.I., C.C. and S.A. prepared the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
The file contains Supplementary Figures S1-S7 with Legends (PDF 2817 kb)
Rights and permissions
About this article
Cite this article
Ishii, K., Kawagoe, T., Koyama, S. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008). https://doi.org/10.1038/nature06537
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06537
This article is cited by
-
NINJ1 mediates inflammatory cell death, PANoptosis, and lethality during infection conditions and heat stress
Nature Communications (2024)
-
Maternal antibodies induced by a live attenuated vaccine protect neonatal mice from cytomegalovirus
npj Vaccines (2023)
-
DNA based neoepitope vaccination induces tumor control in syngeneic mouse models
npj Vaccines (2023)
-
Understanding nucleic acid sensing and its therapeutic applications
Experimental & Molecular Medicine (2023)
-
Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.