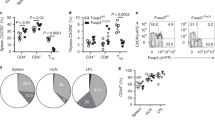
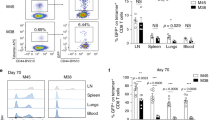
Abstract
A hallmark of adaptive immunity is the generation of memory T cells that confer long-lived, antigen-specific protection against repeat challenges by pathogens1,2,3,4,5. Understanding the mechanisms by which memory T cells arise is important for rational vaccination strategies and improved therapeutic interventions for chronic infections and autoimmune disorders. The large clonal expansion of CD8 T cells in response to some infections has made the development of CD8 T-cell memory more amenable to study, giving rise to a model of memory cell differentiation in which a fraction of fully competent effector T cells transition into long-lived memory T cells4,6,7. Delineation of CD4 T-cell memory development has proved more difficult as a result of limitations on tracking the smaller populations of CD4 effector T cells generated during a pathogenic challenge8,9,10, complicating efforts to determine whether CD4 memory T cells are direct descendants of effector T cells or whether they develop by alternative pathways3,4. Here, using two complementary cytokine reporter mouse models to identify interferon (IFN)-γ-positive effector T cells and track their fate, we show that the lineage relationship between effector and memory CD4 T cells resembles that for CD8 T cells responding to the same pathogen. We find that, in parallel with effector CD8 T cells, IFN-γ-positive effector CD4 T cells give rise to long-lived memory T cells capable of anamnestic responses to antigenic rechallenge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sprent, J. & Surh, C. D. T cell memory. Annu. Rev. Immunol. 20, 551–579 (2002)
Bevan, M. J. Immunology: remembrance of things past. Nature 420, 748–749 (2002)
Kaech, S. M., Wherry, E. J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nature Rev. Immunol. 2, 251–262 (2002)
Seder, R. A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature Immunol. 4, 835–842 (2003)
Foulds, K. E., Wu, C. Y. & Seder, R. A. Th1 memory: implications for vaccine development. Immunol. Rev. 211, 58–66 (2006)
Jacob, J. & Baltimore, D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature 399, 593–597 (1999)
Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002)
Homann, D., Teyton, L. & Oldstone, M. B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nature Med. 7, 913–919 (2001)
Varga, S. M. & Welsh, R. M. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J. Immunol. 161, 367–374 (1998)
Whitmire, J. K., Asano, M. S., Murali-Krishna, K., Suresh, M. & Ahmed, R. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J. Virol. 72, 8281–8288 (1998)
Hatton, R. D. et al. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity 25, 717–729 (2006)
Butz, E. A. & Bevan, M. J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998)
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998)
Fuller, M. J. et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174, 5926–5930 (2005)
Li, J., Huston, G. & Swain, S. L. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198, 1807–1815 (2003)
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999)
Lenz, D. C. et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl Acad. Sci. USA 101, 9357–9362 (2004)
Kondrack, R. M. et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198, 1797–1806 (2003)
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003)
Bachmann, M. F., Wolint, P., Schwarz, K., Jager, P. & Oxenius, A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor α and CD62L. J. Immunol. 175, 4686–4696 (2005)
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001)
Oxenius, A. et al. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur. J. Immunol. 25, 3402–3411 (1995)
Pamer, E. G. Immune responses to Listeria monocytogenes. Nature Rev. Immunol. 4, 812–823 (2004)
Foulds, K. E. et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168, 1528–1532 (2002)
Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001)
Wherry, E. J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 4, 225–234 (2003)
Wu, C. Y. et al. Distinct lineages of TH1 cells have differential capacities for memory cell generation in vivo. Nature Immunol. 3, 852–858 (2002)
Saparov, A. et al. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity 11, 271–280 (1999)
Swain, S. L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999)
Hataye, J., Moon, J. J., Khoruts, A., Reilly, C. & Jenkins, M. K. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312, 114–116 (2006)
Yang, X. W., Model, P. & Heintz, N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nature Biotechnol. 15, 859–865 (1997)
Yu, D. et al. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl Acad. Sci. USA 97, 5978–5983 (2000)
Kontgen, F., Suss, G., Stewart, C., Steinmetz, M. & Bluethmann, H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int. Immunol. 5, 957–964 (1993)
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005)
Fuller, M. J. et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174, 5926–5930 (2005)
Harrington, L. E., Galvan, M., Baum, L. G., Altman, J. D. & Ahmed, R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 191, 1241–1246 (2000)
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998)
Foulds, K. E. et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168, 1528–1532 (2002)
Acknowledgements
We thank R. Hatton and C. Maynard, and other members of the Weaver laboratory, for comments and suggestions; T. Ryan and the UAB ES/Transgenic facility for assistance with blastocyst and embryo injections and the UAB Digestive Diseases Research Development Center (DDRDC) for generation of reporter mice; H. Shen for the provision of LM-OVA; C. Song, H. Turner and M. Blake for technical assistance; and N. LeLievre for editorial assistance. This study was supported in part by a Postdoctoral Fellowship and Career Transition Award from the National Multiple Sclerosis Society (L.E.H.) and by grants from the National Institutes of Health (C.T.W. and A.J.Z.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S6 with Legends. (PDF 3803 kb)
Rights and permissions
About this article
Cite this article
Harrington, L., Janowski, K., Oliver, J. et al. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 452, 356–360 (2008). https://doi.org/10.1038/nature06672
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06672
This article is cited by
-
IFNγ drives neuroinflammation, demyelination, and neurodegeneration in a mouse model of multiple system atrophy
Acta Neuropathologica Communications (2024)
-
Enterocyte–innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium
Mucosal Immunology (2022)
-
The functions of IL-23 and IL-2 on driving autoimmune effector T-helper 17 cells into the memory pool in dry eye disease
Mucosal Immunology (2021)
-
Revisiting cellular immune response to oncogenic Marek’s disease virus: the rising of avian T-cell immunity
Cellular and Molecular Life Sciences (2020)
-
Transcriptome dynamics of CD4+ T cells during malaria maps gradual transit from effector to memory
Nature Immunology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.