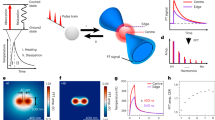
Abstract
Fluorescence, that is, spontaneous emission, is generally more sensitive than absorption measurement, and is widely used in optical imaging1,2. However, many chromophores, such as haemoglobin and cytochromes, absorb but have undetectable fluorescence because the spontaneous emission is dominated by their fast non-radiative decay3. Yet the detection of their absorption is difficult under a microscope. Here we use stimulated emission, which competes effectively with the nonradiative decay, to make the chromophores detectable, and report a new contrast mechanism for optical microscopy. In a pump–probe experiment, on photoexcitation by a pump pulse, the sample is stimulated down to the ground state by a time-delayed probe pulse, the intensity of which is concurrently increased. We extract the miniscule intensity increase with shot-noise-limited sensitivity by using a lock-in amplifier and intensity modulation of the pump beam at a high megahertz frequency. The signal is generated only at the laser foci owing to the nonlinear dependence on the input intensities, providing intrinsic three-dimensional optical sectioning capability. In contrast, conventional one-beam absorption measurement exhibits low sensitivity, lack of three-dimensional sectioning capability, and complication by linear scattering of heterogeneous samples. We demonstrate a variety of applications of stimulated emission microscopy, such as visualizing chromoproteins, non-fluorescent variants of the green fluorescent protein, monitoring lacZ gene expression with a chromogenic reporter, mapping transdermal drug distributions without histological sectioning, and label-free microvascular imaging based on endogenous contrast of haemoglobin. For all these applications, sensitivity is orders of magnitude higher than for spontaneous emission or absorption contrast, permitting nonfluorescent reporters for molecular imaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pawley, J. B. Handbook of Biological Confocal Microscopy 3rd edn (Springer, 2006)
Lakowicz, J. R. Principles of Fluorescence Spectroscopy (Plenum Press, 1983)
Turro, N. J. Modern Molecular Photochemistry (University Science Books, 1991)
Einstein, A. On the quantum theory of radiation. Phys. Z. 18, 121 (1917)
Seigman, A. E. Laser 264–307 (University Science Books, 1986)
Hamilton, C. E., Kinsey, J. L. & Field, R. W. Stimulated emission pumping: new methods in spectroscopy and molecular dynamics. Annu. Rev. Phys. Chem. 37, 493–524 (1986)
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994)
Dong, C. Y., So, P. T., French, T. & Gratton, E. Fluorescence lifetime imaging by asynchronous pump-probe microscopy. Biophys. J. 69, 2234–2242 (1995)
Cantor, C. R. & Schimmel, P. R. Biophysical Chemistry 361–374 (W. H. Freeman, 1980)
Moerner, W. E. & Kador, L. Optical detection and spectroscopy of single molecules in a solid. Phys. Rev. Lett. 62, 2535–2538 (1989)
Ye, J., Ma, L. S. & Hall, J. L. Ultrasensitive detections in atomic and molecular physics: demonstration in molecular overtone spectroscopy. J. Opt. Soc. Am. B 15, 6–15 (1998)
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008)
Fu, D. et al. High-resolution in vivo imaging of blood vessels without labeling. Opt. Lett. 32, 2641–2643 (2007)
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990)
Evans, C. L. & Xie, X. S. Coherent anti-Stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 1, 883–909 (2008)
Rittweger, E., Rankin, B. R., Westphal, V. & Hell, S. W. Fluorescence depletion mechanisms in super-resolving STED microscopy. Chem. Phys. Lett. 442, 483–487 (2007)
Du, H. et al. PhotochemCAD: A computer-aided design and research tool in photochemistry. Photochem. Photobiol. 68, 141–142 (1998)
Gurskaya, N. G. et al. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Lett. 507, 16–20 (2001)
Chan, M. C. Y. et al. Structural characterization of a blue chromoprotein and its yellow mutant from the sea anemone Cnidopus japonicus . J. Biol. Chem. 281, 37813–37819 (2006)
Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. Creating new fluorescent probes for cell biology. Nature Rev. Mol. Biol. 3, 906–918 (2002)
Miller, J. H. Experiments in Molecular Genetics 171–224 (Cold Spring Harbor Laboratory, 1972)
Cai, L., Friedman, N. & Xie, X. S. Stochastic protein expression in individual cells at the single molecule level. Nature 440, 358–362 (2006)
Tremblay, J. F. et al. Photodynamic therapy with toluidine blue in Jurkat cells: cytotoxicity, subcellular localization and apoptosis induction. Photochem. Photobiol. Sci. 1, 852–856 (2002)
Chelvanayagam, D. K. & Beazley, L. D. Toluidine blue-O is a Nissl bright-field counterstain for lipophilic fluorescent tracers Di-ASP, DiI and DiO. J. Neurosci. Methods 72, 49–55 (1997)
McDonald, D. M. & Choyke, P. L. Imaging of angiogenesis: from microscope to clinic. Nature Med. 9, 713–725 (2003)
Grinvald, A., Lieke, E., Frostig, R. D., Gilbert, C. D. & Wiesel, T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324, 361–364 (1986)
Kleinfeld, D., Mitra, P. P., Helmchen, F. & Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl Acad. Sci. USA 95, 15741–15746 (1998)
Clay, G. O., Schaffer, C. B. & Kleinfeld, D. Large two-photon absorptivity of hemoglobin in the infrared range of 780–880 nm. J. Chem. Phys. 126, 025102 (2007)
Wang, W. et al. Femtosecond multicolor pump-probe spectroscopy of ferrous cytochrome c. J. Phys. Chem. B 104, 10789–10801 (2000)
Acknowledgements
We thank K. Lukyanov and A. Miyawaki for the gifts of chromoprotein gtCP and cjBlue plasmid DNA, respectively; Coherent Inc. for lending us a femtosecond optical parametric oscillator; and P. Choi for preparing X-gal E. coli cells. We also thank B. G. Saar, C. W. Freudiger, S. Basu, J. W. Lichtman and C. B. Schaffer for discussions, and R. Tsien for suggesting the use of chromoproteins. This work was supported by a National Science Foundation (grant CHE-0634788) and the US Department of Energy’s Basic Energy Sciences Program (DE-FG02-07ER15875).
Author Contributions W.M., S.L. and S.C. performed experiments and analysed data. R.R. constructed E. coli cells expressing chromoproteins. G.R.H. and S.C. helped to construct the laser systems. W.M., S.L. and X.S.X. conceived the concept, designed the experiments and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
[Competing interests: Harvard University has recently filed a US patent application (“Systems and Methods for Stimulated Emission Imaging.”) on behalf of X.S.X., W.M. and S.L. based on this work.]
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Supplementary Figures 1-3 with Legends. (PDF 513 kb)
Rights and permissions
About this article
Cite this article
Min, W., Lu, S., Chong, S. et al. Imaging chromophores with undetectable fluorescence by stimulated emission microscopy. Nature 461, 1105–1109 (2009). https://doi.org/10.1038/nature08438
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08438
This article is cited by
-
Longitudinal piezoelectric resonant photoelastic modulator for efficient intensity modulation at megahertz frequencies
Nature Communications (2022)
-
Advances in nonlinear optical microscopy techniques for in vivo and in vitro neuroimaging
Biophysical Reviews (2021)
-
Stimulated Raman excited fluorescence spectroscopy and imaging
Nature Photonics (2019)
-
Room-temperature ultrafast nonlinear spectroscopy of a single molecule
Nature Photonics (2018)
-
Resolution enhancement of pump–probe microscope with an inverse-annular filter
Optical Review (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.