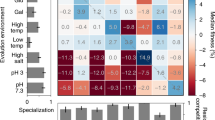
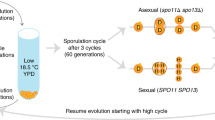
Abstract
The tendency of organisms to reproduce by cross-fertilization despite numerous disadvantages relative to self-fertilization is one of the oldest puzzles in evolutionary biology. For many species, the primary obstacle to the evolution of outcrossing is the cost of production of males1, individuals that do not directly contribute offspring and thus diminish the long-term reproductive output of a lineage. Self-fertilizing (‘selfing’) organisms do not incur the cost of males and therefore should possess at least a twofold numerical advantage over most outcrossing organisms2. Two competing explanations for the widespread prevalence of outcrossing in nature despite this inherent disadvantage are the avoidance of inbreeding depression generated by selfing3,4,5 and the ability of outcrossing populations to adapt more rapidly to environmental change1,6,7. Here we show that outcrossing is favoured in populations of Caenorhabditis elegans subject to experimental evolution both under conditions of increased mutation rate and during adaptation to a novel environment. In general, fitness increased with increasing rates of outcrossing. Thus, each of the standard explanations for the maintenance of outcrossing are correct, and it is likely that outcrossing is the predominant mode of reproduction in most species because it is favoured under ecological conditions that are ubiquitous in natural environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Maynard Smith, J. The Evolution of Sex (Cambridge Univ. Press, 1978)
Lively, C. M. & Lloyd, D. G. The cost of biparental sex under individual selection. Am. Nat. 135, 489–500 (1990)
Charlesworth, D. & Charlesworth, B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268 (1987)
Lande, R. & Schemske, D. W. The evolution of self-fertilization and inbreeding depression in plants. 1. Genetic models. Evolution 39, 24–40 (1985)
Heller, J. & Maynard Smith, J. Does Muller's Ratchet work with selfing? Genet. Res. 8, 269–294 (1979)
Stebbins, G. L. Self-fertilization and population variation in higher plants. Am. Nat. 91, 337–354 (1957)
Crow, J. F. An advantage of sexual reproduction in a rapidly changing environment. J. Hered. 83, 169–173 (1992)
Fisher, R. A. Average excess and average effect of a gene substitution. Ann. Eugen. 11, 53–63 (1941)
Williams, G. C. Sex and Evolution (Princeton Univ. Press, 1975)
Kondrashov, A. S. Deleterious mutations as an evolutionary factor. I. The advantage of recombination. Genet. Res. 44, 199–217 (1984)
Schultz, S. T. & Lynch, M. Mutation and extinction: the role of variable mutational effects, synergistic epistasis, beneficial mutations, and degree of outcrossing. Evolution 51, 1363–1371 (1997)
Felsenstein, J. The evolutionary advantage of recombination. Genetics 78, 737–756 (1974)
Barton, N. H. Linkage and the limits to natural selection. Genetics 140, 821–841 (1995)
Chasnov, J. R. & Chow, K. L. Why are there males in the hermaphroditic species Caenorhabditis elegans? Genetics 160, 983–994 (2002)
Stewart, A. D. & Phillips, P. C. Selection and maintenance of androdioecy in Caenorhabditis elegans . Genetics 160, 975–982 (2002)
Sivasundar, A. & Hey, J. Population genetics of Caenorhabditis elegans: the paradox of low polymorphism in a widespread species. Genetics 163, 147–157 (2003)
Barriere, A. & Felix, M. A. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr. Biol. 15, 1176–1184 (2005)
Haber, M. et al. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Mol. Biol. Evol. 22, 160–173 (2005)
Teotónio, H., Manoel, D. & Phillips, P. C. Genetic variation for outcrossing among Caenorhabditis elegans isolates. Evolution 60, 1300–1305 (2006)
Miller, L. M., Plenefisch, J. D., Casson, L. P. & Meyer, B. J. xol-1 — a gene that controls the male modes of both sex determination and X-chromosome dosage compensation in C. elegans . Cell 55, 167–183 (1988)
Schedl, T. & Kimble, J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans . Genetics 119, 43–61 (1988)
Estes, S., Phillips, P. C., Denver, D. R., Thomas, W. K. & Lynch, M. Mutation accumulation in populations of varying size: the distribution of mutational effects for fitness correlates in Caenorhabditis elegans . Genetics 166, 1269–1279 (2004)
Loewe, L. & Cutter, A. D. On the potential for extinction by Muller's ratchet in Caenorhabditis elegans . BMC Evol. Biol. 8, 125 (2008)
Cutter, A. D. Mutation and the experimental evolution of outcrossing in Caenorhabditis elegans . J. Evol. Biol. 18, 27–34 (2005)
Manoel, D., Carvalho, S., Phillips, P. C. & Teotónio, H. Selection against males in Caenorhabditis elegans under two mutational treatments. Proc. R. Soc. Lond. B 274, 417–424 (2007)
Pradel, E. et al. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans . Proc. Natl Acad. Sci. USA 104, 2295–2300 (2007)
Mallo, G. V. et al. Inducible antibacterial defense system in C. elegans . Curr. Biol. 12, 1209–1214 (2002)
Colegrave, N. Sex releases the speed limit on evolution. Nature 420, 664–666 (2002)
Goddard, M. R., Charles, H., Godfray, J. & Burt, A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434, 636–640 (2005)
Whitlock, M. C. & Agrawal, A. F. Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63, 569–582 (2009)
Acknowledgements
We thank S. Scholz, A. Ohdera and J. Chiem for logistical help, S. Katz for providing the S. marcescens 2170 strain, and J. Thornton for use of laboratory space and equipment. We also thank B. Cresko, C. Lively, J. Thornton and the members of the Phillips and Cresko laboratories for comments and discussion pertaining to this work. Funding was provided by NSF grants DEB-0236180, DEB-0710386 and DEB-0641066, and an NIH Genetics Fellowship awarded to L.T.M. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Author Contributions L.T.M. and P.C.P. designed the experiments. L.T.M. and M.D.P. performed the experiments. L.T.M. and P.C.P. analysed the data. L.T.M. and P.C.P. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Table S1, Supplementary References and Supplementary Figures S1-S2 with Legends. (PDF 1362 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Morran, L., Parmenter, M. & Phillips, P. Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature 462, 350–352 (2009). https://doi.org/10.1038/nature08496
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08496
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.