Abstract
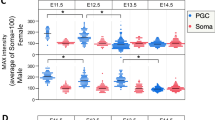
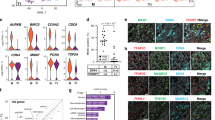
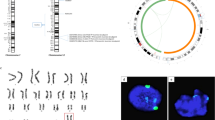
The leading cause of infertility in men and women is quantitative and qualitative defects in human germ-cell (oocyte and sperm) development. Yet, it has not been possible to examine the unique developmental genetics of human germ-cell formation and differentiation owing to inaccessibility of germ cells during fetal development. Although several studies have shown that germ cells can be differentiated from mouse and human embryonic stem cells, human germ cells differentiated in these studies generally did not develop beyond the earliest stages1,2,3,4,5,6,7,8. Here we used a germ-cell reporter to quantify and isolate primordial germ cells derived from both male and female human embryonic stem cells. By silencing and overexpressing genes that encode germ-cell-specific cytoplasmic RNA-binding proteins (not transcription factors), we modulated human germ-cell formation and developmental progression. We observed that human DAZL (deleted in azoospermia-like) functions in primordial germ-cell formation, whereas closely related genes DAZ and BOULE (also called BOLL) promote later stages of meiosis and development of haploid gametes. These results are significant to the generation of gametes for future basic science and potential clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
12 November 2009
A definition for the scale bars in the legend for Fig. 2b was added on 12 November 2009.
References
Hubner, K. et al. Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256 (2003)
Toyooka, Y., Tsunekawa, N., Akasu, R. & Noce, T. Embryonic stem cells can form germ cells in vitro. Proc. Natl Acad. Sci. USA 100, 11457–11462 (2003)
Geijsen, N. et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427, 148–154 (2004)
Clark, A. T. et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 13, 727–739 (2004)
Kee, K., Gonsalves, J. M., Clark, A. T. & Reijo Pera, R. A. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 15, 831–837 (2006)
Tilgner, K. et al. Isolation of primoridal germ cells from differentiating human embryonic stem cells. Stem Cells 26, 3075–3085 (2008)
Bucay, N. et al. A novel approach for the derivation of putative primordial germ cells and Sertoli cells from human embryonic stem cells. Stem Cells 27, 68–77 (2009)
Park, T. S. et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 27, 783–795 (2009)
Hull, M. G. R. et al. Population study of causes, treatment, and outcome of infertility. Br. Med. J. (Clin. Res. Ed.) 291, 1693–1697 (1985)
Xu, E. Y., Moore, F. L. & Reijo Pera, R. A. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc. Natl Acad. Sci. USA 98, 7414–7419 (2001)
Fujiwara, Y. et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl Acad. Sci. USA 91, 12258–12262 (1994)
Castrillon, D. H., Quade, B. J., Wang, T. Y., Quigley, C. & Crum, C. P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl Acad. Sci. USA 97, 9585–9590 (2000)
Zhao, G. Q. Consequences of knocking out BMP signaling in the mouse. Genesis 35, 43–56 (2003)
Mahadevaiah, S. K. et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nature Genet. 27, 271–276 (2001)
Lenzi, M. L. et al. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis I in human oocytes. Am. J. Hum. Genet. 76, 112–127 (2005)
Heyting, C. Synaptonemal complexes: structure and function. Curr. Opin. Cell Biol. 8, 389–396 (1996)
Perrett, R. M. et al. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol. Reprod. 78, 852–858 (2008)
Miyamoto, T. et al. Isolation and expression analysis of the testis-specific gene, STRA8, stimulated by retinoic acid gene 8. J. Assist. Reprod. Genet. 19, 531–535 (2002)
Hajkova, P. et al. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 117, 15–23 (2002)
West, J. A. et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460, 909–913 (2009)
Shamblott, M. J. et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726–13731 (1998)
McLaren, A. Primordial germ cells in the mouse. Dev. Biol. 262, 1–15 (2003)
Rugg-Gunn, P. J., Ferguson-Smith, A. C. & Pedersen, R. A. Epigenetic status of human embryonic stem cells. Nature Genet. 37, 585–587 (2005)
Reijo, R. et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nature Genet. 10, 383–393 (1995)
Reijo, R., Alagappan, R. K., Patrizio, P. & Page, D. C. Severe oligospermia resulting from deletions of the Azoospermia Factor gene on the Y chromosome. Lancet 347, 1290–1293 (1996)
Flörke-Gerloff, S., Topfer-Petersen, E., Muller-Esterl, W., Schill, W. B. & Engel, W. Acrosin and the acrosome in human spermatogenesis. Hum. Genet. 65, 61–67 (1983)
Suter, D. M. et al. Rapid generation of stable transgenic embryonic stem cell lines using modular lentivectors. Stem Cells 24, 615–623 (2006)
Reijo, R. A. et al. DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol. Reprod. 63, 1490–1496 (2000)
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002)
Kerjean, A. et al. Establishment of the paternal methylation imprint of the human H19 and MEST/PEG1 genes during spermatogenesis. Hum. Mol. Genet. 9, 2183–2187 (2000)
Oakes, C. C., La Salle, S., Robaire, B. & Trasler, J. M. Evaluation of a quantitative DNA methylation analysis technique using methylation-sensitive/dependent restriction enzymes and real-time PCR. Epigenetics 1, 146–152 (2006)
Gonsalves, J. et al. Defective recombination in infertile men. Hum. Mol. Genet. 13, 2875–2883 (2004)
Darzynkiewicz, Z. & Juan, G. DNA content measurement for DNA ploidy and cell cycle analysis. Curr. Protoc. Cytom. Ch. 7, Unit 7 5 (2001)
Acknowledgements
We thank D. Suter for the p2k7 vectors, B. Behr for procurement of clinical samples, C. Nicholas for assistance with SCP3 staining, and S. Jiang and P. Lovelace for FACS expertise. K.K. was supported by a California TRDRP postdoctoral fellowship; V.T.A. was supported by an NIH fellowship. This work was supported by the National Institutes of Health (RO1HD047721, U54HD055764 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research), the California TRDRP (14RT0159) and the California Institute for Regenerative Medicine (RC1-00137) to R.A.R.P.
Author Contributions K.K. carried out the majority of experiments with assistance with imprint analysis and gene expression analysis by V.T.A., silencing of BOULE and DAZ by M.F., and 5mC and FISH staining by H.N.N.; K.K. and R.A.R.P. designed experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-22 with Legends. (PDF 1917 kb)
Rights and permissions
About this article
Cite this article
Kee, K., Angeles, V., Flores, M. et al. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation . Nature 462, 222–225 (2009). https://doi.org/10.1038/nature08562
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08562
This article is cited by
-
Modelling in vitro gametogenesis using induced pluripotent stem cells: a review
Cell Regeneration (2023)
-
DMRT1 drives the human germline forward
Nature Cell Biology (2023)
-
Sublethal effects of acetaminophen exposure on benthic aquatic animal (Hydra magnipapillata)
Molecular & Cellular Toxicology (2023)
-
In vitro differentiation of primed human induced pluripotent stem cells into primordial germ cell-like cells
Molecular Biology Reports (2023)
-
Germline specification from pluripotent stem cells
Stem Cell Research & Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.