Abstract
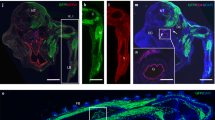
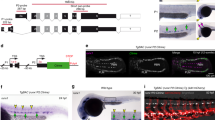
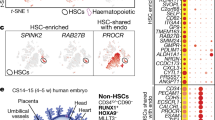
A major goal of regenerative medicine is to instruct formation of multipotent, tissue-specific stem cells from induced pluripotent stem cells (iPSCs) for cell replacement therapies. Generation of haematopoietic stem cells (HSCs) from iPSCs or embryonic stem cells (ESCs) is not currently possible, however, necessitating a better understanding of how HSCs normally arise during embryonic development. We previously showed that haematopoiesis occurs through four distinct waves during zebrafish development, with HSCs arising in the final wave in close association with the dorsal aorta. Recent reports have suggested that murine HSCs derive from haemogenic endothelial cells (ECs) lining the aortic floor1,2. Additional in vitro studies have similarly indicated that the haematopoietic progeny of ESCs arise through intermediates with endothelial potential3,4. Here we have used the unique strengths of the zebrafish embryo to image directly the generation of HSCs from the ventral wall of the dorsal aorta. Using combinations of fluorescent reporter transgenes, confocal time-lapse microscopy and flow cytometry, we have identified and isolated the stepwise intermediates as aortic haemogenic endothelium transitions to nascent HSCs. Finally, using a permanent lineage tracing strategy, we demonstrate that the HSCs generated from haemogenic endothelium are the lineal founders of the adult haematopoietic system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zovein, A. C. et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625–636 (2008)
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E. & Speck, N. A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891 (2009)
Eilken, H. M., Nishikawa, S. & Schroeder, T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900 (2009)
Lancrin, C. et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895 (2009)
Cumano, A. & Godin, I. Ontogeny of the hematopoietic system. Annu. Rev. Immunol. 25, 745–785 (2007)
Murry, C. E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008)
de Bruijn, M. F., Speck, N. A., Peeters, M. C. & Dzierzak, E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465–2474 (2000)
Dzierzak, E. & Speck, N. A. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature Immunol. 9, 129–136 (2008)
Bertrand, J. Y., Kim, A. D., Teng, S. & Traver, D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development 135, 1853–1862 (2008)
North, T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007)
Chi, N. C. et al. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739 (2008)
Kissa, K. et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood 111, 1147–1156 (2008)
Huang, H., Zhang, B., Hartenstein, P. A., Chen, J. N. & Lin, S. NXT2 is required for embryonic heart development in zebrafish. BMC Dev. Biol. 5, 7 (2005)
Mikkola, H. K., Fujiwara, Y., Schlaeger, T. M., Traver, D. & Orkin, S. H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101, 508–516 (2003)
Bertrand, J. Y. et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc. Natl Acad. Sci. USA 102, 134–139 (2005)
Jin, S. W., Beis, D., Mitchell, T., Chen, J. N. & Stainier, D. Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005)
Bussmann, J., Bakkers, J. & Schulte-Merker, S. Early endocardial morphogenesis requires Scl/Tal1. PLoS Genet. 3, e140 (2007)
Liao, W. et al. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development 124, 381–389 (1997)
Choi, J. et al. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev. Biol. 304, 735–744 (2007)
Traver, D. et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nature Immunol. 4, 1238–1246 (2003)
Jaffredo, T., Gautier, R., Eichmann, A. & Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125, 4575–4583 (1998)
Ciau-Uitz, A., Walmsley, M. & Patient, R. Distinct origins of adult and embryonic blood in Xenopus . Cell 102, 787–796 (2000)
Feng, H. et al. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br. J. Haematol. 138, 169–175 (2007)
Westerfield, M. The zebrafish book: A guide for the laboratory use of zebrafish (Brachydanio rerio) 2.1 edn (Univ. of Oregon Press, 1994)
Beis, D. et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132, 4193–4204 (2005)
MacPherson, D. et al. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 18, 1681–1694 (2004)
Kawakami, K. et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 (2004)
Rozen, S. & Skaletsky, H. J. Primer3 on the WWW for general users and biologist programmers. Methods Mol. Biol. 132, 365–386 (2000)
Acknowledgements
We thank S. Lin for providing kdrl:RFP animals. J.Y.B. was supported by the Irvington program of the Cancer Research Institute and by the California Institute for Regenerative Medicine (CIRM), N.C.C. by National Institutes of Health (NIH) HL074891, a Research and Education Foundation Award from GlaxoSmithKline and a Beginning Grant in Aid Award from the American Heart Association, B.S. by NIH F32DK752433, D.Y.R.S. by the Packard Foundation and NIH HL54737, and D.T. by a Scholar Award from the American Society of Hematology, a New Investigator Award from CIRM, and NIH DK074482.
Author Contributions J.Y.B., N.C.C. and D.T. designed experiments. J.Y.B. and D.T. wrote the manuscript, with key input from N.C.C. and D.Y.R.S.; J.Y.B. performed experiments. B.S. and S.T. generated and characterized the bactin:switch reporter line. N.C.C. and D.Y.R.S. generated kdrl:Cre and kdrl:memCherry transgenic lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-3 with Legends. (PDF 2510 kb)
Supplementary Movie 1
This movie shows emergence of HSCs from the floor of the dorsal aorta. (MOV 5321 kb)
Supplementary Movie 2
This movie shows close up of HSC emergence. (MOV 6540 kb)
Rights and permissions
About this article
Cite this article
Bertrand, J., Chi, N., Santoso, B. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010). https://doi.org/10.1038/nature08738
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08738
This article is cited by
-
Exploring hematopoiesis in zebrafish using forward genetic screening
Experimental & Molecular Medicine (2024)
-
Cis inhibition of NOTCH1 through JAGGED1 sustains embryonic hematopoietic stem cell fate
Nature Communications (2024)
-
Generating hematopoietic cells from human pluripotent stem cells: approaches, progress and challenges
Cell Regeneration (2023)
-
Physiology and diseases of tissue-resident macrophages
Nature (2023)
-
Alcam-a and Pdgfr-α are essential for the development of sclerotome-derived stromal cells that support hematopoiesis
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.