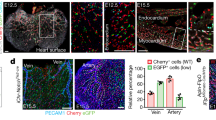
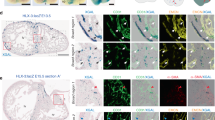
Abstract
Coronary artery disease is the leading cause of death worldwide. Determining the coronary artery developmental program could aid understanding of the disease and lead to new treatments, but many aspects of the process, including their developmental origin, remain obscure. Here we show, using histological and clonal analysis in mice and cardiac organ culture, that coronary vessels arise from angiogenic sprouts of the sinus venosus—the vein that returns blood to the embryonic heart. Sprouting venous endothelial cells dedifferentiate as they migrate over and invade the myocardium. Invading cells differentiate into arteries and capillaries; cells on the surface redifferentiate into veins. These results show that some differentiated venous cells retain developmental plasticity, and indicate that position-specific cardiac signals trigger their dedifferentiation and conversion into coronary arteries, capillaries and veins. Understanding this new reprogramming process and identifying the endogenous signals should suggest more natural ways of engineering coronary bypass grafts and revascularizing the heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Folkman, J. & Haudenschild, C. Angiogenesis in vitro . Nature 288, 551–556 (1980)
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989)
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005)
Adams, R. H. & Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature Rev. Mol. Cell Biol. 8, 464–478 (2007)
Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005)
Folkman, J. Angiogenesis. Annu. Rev. Med. 57, 1–18 (2006)
Majesky, M. W. Development of coronary vessels. Curr. Top. Dev. Biol. 62, 225–259 (2004)
Lavine, K. J. & Ornitz, D. M. Shared circuitry: developmental signaling cascades regulate both embryonic and adult coronary vasculature. Circ. Res. 104, 159–169 (2009)
Smart, N., Dube, K. N. & Riley, P. R. Coronary vessel development and insight towards neovascular therapy. Int. J. Exp. Pathol. 90, 262–283 (2009)
World Health Organization. The Global Burden Of Disease: 2004 Update 〈http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html〉.
Lewis, F. T. The question of sinusoids. Anat. Anz. 25, 261–279 (1904)
Grant, R. T. Development of the cardiac coronary vessels in the rabbit. Heart 13, 261–271 (1923)
Bennett, H. S. The development of the blood supply to the heart in the embryo pig. Am. J. Anat. 60, 27–53 (1936)
Hutchins, G. M., Kessler-Hanna, A. & Moore, G. W. Development of the coronary arteries in the embryonic human heart. Circulation 77, 1250–1257 (1988)
Mikawa, T. & Gourdie, R. G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 174, 221–232 (1996)
Männer, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 255, 212–226 (1999)
Pérez-Pomares, J. M. et al. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 46, 1005–1013 (2002)
Kirby, M. L. Cardiac Development (Oxford Univ. Press, 2007)
Poelmann, R. E., Gittenberger-de Groot, A. C., Mentink, M. M., Bokenkamp, R. & Hogers, B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 73, 559–568 (1993)
Merki, E. et al. Epicardial retinoid X receptor α is required for myocardial growth and coronary artery formation. Proc. Natl Acad. Sci. USA 102, 18455–18460 (2005)
Wilm, B., Ipenberg, A., Hastie, N. D., Burch, J. B. & Bader, D. M. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132, 5317–5328 (2005)
Cai, C. L. et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 (2008)
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008)
Sheikh, A. Y. et al. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am. J. Physiol. Heart Circ. Physiol. 294, H88–H98 (2008)
Kattan, J., Dettman, R. W. & Bristow, J. Formation and remodeling of the coronary vascular bed in the embryonic avian heart. Dev. Dyn. 230, 34–43 (2004)
Lavine, K. J. et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 20, 1651–1666 (2006)
Hiruma, T. & Hirakow, R. Epicardial formation in embryonic chick heart: computer-aided reconstruction, scanning, and transmission electron microscopic studies. Am. J. Anat. 184, 129–138 (1989)
Hellström, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007)
Kidoya, H. et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 27, 522–534 (2008)
Tammela, T. et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 (2008)
Suchting, S. et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl Acad. Sci. USA 104, 3225–3230 (2007)
Drake, C. J. & Fleming, P. A. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95, 1671–1679 (2000)
Monvoisin, A. et al. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev. Dyn. 235, 3413–3422 (2006)
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007)
Lavine, K. J., Long, F., Choi, K., Smith, C. & Ornitz, D. M. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development 135, 3161–3171 (2008)
Swift, M. R. & Weinstein, B. M. Arterial-venous specification during development. Circ. Res. 104, 576–588 (2009)
le Noble, F. et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 131, 361–375 (2004)
Moyon, D., Pardanaud, L., Yuan, L., Breant, C. & Eichmann, A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development 128, 3359–3370 (2001)
Othman-Hassan, K. et al. Arterial identity of endothelial cells is controlled by local cues. Dev. Biol. 237, 398–409 (2001)
Kudo, F. A. et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler. Thromb. Vasc. Biol. 27, 1562–1571 (2007)
Goldman, S. et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 44, 2149–2156 (2004)
Sabik, J. F., Lytle, B. W., Blackstone, E. H., Houghtaling, P. L. & Cosgrove, D. M. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann. Thorac. Surg. 79, 544–551,discussion 544–551 (2005)
Metzger, R. J., Klein, O. D., Martin, G. R. & Krasnow, M. A. The branching programme of mouse lung development. Nature 453, 745–750 (2008)
Hayashi, S. & McMahon, A. P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 (2002)
Zovein, A. C. et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625–636 (2008)
Acknowledgements
We thank T. Quertermous, L. Iruela-Arispe and S. M. Evans for mouse strains, Krasnow laboratory members for input and comments, especially M. Kumar for guidance on clonal analysis, and C. Breitweiser and M. Petersen for help preparing figures. K.R.-H. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (2T32HD007249). M.A.K. is an investigator of the Howard Hughes Medical Institute.
Author Contributions K.R.-H. designed and performed all experiments. K.R.-H. and M.A.K. analysed the experiments and wrote the manuscript. H.U. and I.L.W. provided the multicolour reporter mice and advised on its use.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-10 with legends and Supplementary Table S1. (PDF 25390 kb)
Supplementary Movie 1
This file contains a timelapse video (10 frames/sec) of intact heart cultured for 72 hours. (MOV 2994 kb)
Supplementary Movie 2
This file contains a timelapse video (10 frames/sec) of dissected ventricle cultured for 72 hours. (MOV 550 kb)
Supplementary Movie 3
This file contains a timelapse video (10 frames/sec) of dissected atria cultured for 72 hours. (MOV 248 kb)
Supplementary Movie 4
This file contains a timelapse video (10 frames/sec) of recombined heart cultured for 72 hours. (MOV 2145 kb)
Rights and permissions
About this article
Cite this article
Red-Horse, K., Ueno, H., Weissman, I. et al. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010). https://doi.org/10.1038/nature08873
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08873
This article is cited by
-
Animal models to study cardiac regeneration
Nature Reviews Cardiology (2024)
-
Aorta Without Coronary Arteries: Anatomic Variants of a Rare Malformation
Pediatric Cardiology (2024)
-
Origin of congenital coronary arterio-ventricular fistulae from anomalous epicardial and myocardial development
Experimental & Molecular Medicine (2023)
-
Coronary vessels contribute to de novo endocardial cells in the endocardium-depleted heart
Cell Discovery (2023)
-
Modeling angiogenesis in the human brain in a tissue-engineered post-capillary venule
Angiogenesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.