Abstract
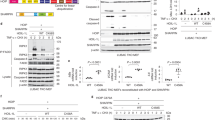
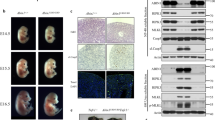
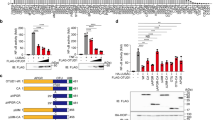
SHARPIN is a ubiquitin-binding and ubiquitin-like-domain-containing protein which, when mutated in mice, results in immune system disorders and multi-organ inflammation1,2. Here we report that SHARPIN functions as a novel component of the linear ubiquitin chain assembly complex (LUBAC) and that the absence of SHARPIN causes dysregulation of NF-κB and apoptotic signalling pathways, explaining the severe phenotypes displayed by chronic proliferative dermatitis (cpdm) in SHARPIN-deficient mice. Upon binding to the LUBAC subunit HOIP (also known as RNF31), SHARPIN stimulates the formation of linear ubiquitin chains in vitro and in vivo. Coexpression of SHARPIN and HOIP promotes linear ubiquitination of NEMO (also known as IKBKG), an adaptor of the IκB kinases (IKKs) and subsequent activation of NF-κB signalling, whereas SHARPIN deficiency in mice causes an impaired activation of the IKK complex and NF-κB in B cells, macrophages and mouse embryonic fibroblasts (MEFs). This effect is further enhanced upon concurrent downregulation of HOIL-1L (also known as RBCK1), another HOIP-binding component of LUBAC. In addition, SHARPIN deficiency leads to rapid cell death upon tumour-necrosis factor α (TNF-α) stimulation via FADD- and caspase-8-dependent pathways. SHARPIN thus activates NF-κB and inhibits apoptosis via distinct pathways in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Grabbe, C. & Dikic, I. Functional roles of ubiquitin-like domain (ULD) and ubiquitin-binding domain (UBD) containing proteins. Chem. Rev. 109, 1481–1494 (2009)
Seymour, R. E. et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 8, 416–421 (2007)
Hayden, M. S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008)
Wertz, I. E. & Dixit, V. M. Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 19, 313–324 (2008)
Ikeda, F. & Dikic, I. Atypical ubiquitin chains: new molecular signals. Review series ‘Protein modifications: beyond the usual suspects’. EMBO Rep. 9, 536–542 (2008)
Iwai, K. & Tokunaga, F. Linear polyubiquitination: a new regulator of NF-κΒ activation. EMBO Rep. 10, 706–713 (2009)
Pasparakis, M. Regulation of tissue homeostasis by NF-κB signalling: implications for inflammatory diseases. Nature Rev. Immunol. 9, 778–788 (2009)
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009)
Kirisako, T. et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 25, 4877–4887 (2006)
Lim, S. et al. Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins. Mol. Cell. Neurosci. 17, 385–397 (2001)
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009)
Wagner, S. et al. Ubiquitin binding mediates the NF-κB inhibitory potential of ABIN proteins. Oncogene 27, 3739–3745 (2008)
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009)
Tokunaga, F. et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nature Cell Biol. 11, 123–132 (2009)
Gijbels, M. J. J., HogenEsch, H., Blauw, B., Roholl, P. & Zurcher, C. Ultrastructure of epidermis of mice with chronic proliferative dermatitis. Ultrastruct. Pathol. 19, 107–111 (1995)
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003)
Wilson, N. S., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nature Immunol. 10, 348–355 (2009)
Dynek, J. N. et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 29, 4198–4209 (2010)
Ikeda, F., Crosetto, N. & Dikic, I. What determines the specificity and outcomes of ubiquitin signaling? Cell 143, 677–681 (2010)
Pickart, C. M. & Raasi, S. Controlled synthesis of polyubiquitin chains. Methods Enzymol. 399, 21–36 (2005)
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols 1, 2856–2860 (2007)
Nielsen, M. L. et al. Iodoacetamide-induced artifact mimics ubiquitination in mass spectrometry. Nature Methods 5, 459–460 (2008)
Ishihama, Y., Rappsilber, J. & Mann, M. Modular stop and go extraction tips with stacked disks for parallel and multidimensional peptide fractionation in proteomics. J. Proteome Res. 5, 988–994 (2006)
Borchert, N. et al. Proteogenomics of Pristionchus pacificus reveals distinct proteome structure of nematode models. Genome Res. 20, 837–846 (2010)
Olsen, J. V. et al. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 (2005)
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnol. 26, 1367–1372 (2008)
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nature Protocols 2, 1896–1906 (2007)
Acknowledgements
We thank E. Kim, K. Rajalingham and H.-J. Kreienkampfor reagents used in this study, I. Matic for initial MS analysis of HOIP/HOIL-1L samples, S. Wahl for sample preparation, and V. Dötsch and members of the Dikic lab for discussions and comments. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DI 931/3-1), the Cluster of Excellence “Macromolecular Complexes” of the Goethe University Frankfurt (EXC115) to I.D., Landesstiftung Baden-Württemberg to B.M., the Medical Research Council UK to K.R. and B.S., JSPS Postdoctoral Fellowships for Research Abroad to F.I., EMBO long-term fellowship to S.S.S. and The National Institutes of Health (AR049288 to J.P.S.). V.N. was supported by the Unity Through Knowledge Fund, 3B Grant. C.G. acknowledges support from The International Human Frontier Science Program Organization.
Author information
Authors and Affiliations
Contributions
F.I., Y.L.D., S.S.S., B.S., C.G., S.J.L.v.W., B.M., V.N., M.F.-W. and P.G. performed the experiments. F.T., A.A. and T.N. contributed with reagents used throughout the study. F.I., Y.L.D., S.S.S., C.G., M.P., J.T., K.I., J.P.S., L.F., B.M. and K.R. contributed to the project by co-ordination of experimental work and writing the manuscript. I.D. provided ideas, co-ordinated the entire project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9 with legends. (PDF 5026 kb)
Rights and permissions
About this article
Cite this article
Ikeda, F., Deribe, Y., Skånland, S. et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471, 637–641 (2011). https://doi.org/10.1038/nature09814
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09814
This article is cited by
-
Mediators of necroptosis: from cell death to metabolic regulation
EMBO Molecular Medicine (2024)
-
LUBAC-mediated M1 Ub regulates necroptosis by segregating the cellular distribution of active MLKL
Cell Death & Disease (2024)
-
LUBAC promotes angiogenesis and lung tumorigenesis by ubiquitinating and antagonizing autophagic degradation of HIF1α
Oncogenesis (2024)
-
LUBAC is required for RIG-I sensing of RNA viruses
Cell Death & Differentiation (2024)
-
Mechanisms underlying linear ubiquitination and implications in tumorigenesis and drug discovery
Cell Communication and Signaling (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.