Abstract
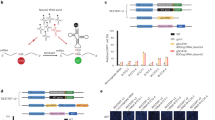
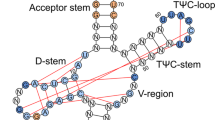
All three translation termination codons, or nonsense codons, contain a uridine residue at the first position of the codon1,2,3. Here, we demonstrate that pseudouridylation (conversion of uridine into pseudouridine (Ψ), ref. 4) of nonsense codons suppresses translation termination both in vitro and in vivo. In vivo targeting of nonsense codons is accomplished by the expression of an H/ACA RNA capable of directing the isomerization of uridine to Ψ within the nonsense codon. Thus, targeted pseudouridylation represents a novel approach for promoting nonsense suppression in vivo. Remarkably, we also show that pseudouridylated nonsense codons code for amino acids with similar properties. Specifically, ΨAA and ΨAG code for serine and threonine, whereas ΨGA codes for tyrosine and phenylalanine, thus suggesting a new mode of decoding. Our results also suggest that RNA modification, as a naturally occurring mechanism, may offer a new way to expand the genetic code.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brenner, S., Barnett, L., Katz, E. R. & Crick, F. H. UGA: a third nonsense triplet in the genetic code. Nature 213, 449–450 (1967)
Brenner, S., Stretton, A. O. & Kaplan, S. Genetic code: the ‘nonsense’ triplets for chain termination and their suppression. Nature 206, 994–998 (1965)
Weigert, M. G. & Garen, A. Base composition of nonsense codons in E. coli. Evidence from amino-acid substitutions at a tryptophan site in alkaline phosphatase. Nature 206, 992–994 (1965)
Cohn, W. E. 5-Ribosyl uracil, a carbon-carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim. Biophys. Acta 32, 569–571 (1959)
Charette, M. & Gray, M. W. Pseudouridine in RNA: what, where, how, and why. IUBMB Life 49, 341051 (2000)
Lesser, C. F. & Guthrie, C. Mutational analysis of pre-mRNA splicing in Saccharomyces cerevisiae using a sensitive new reporter gene, CUP1. Genetics 133, 851–863 (1993)
Hamer, D. H., Thiele, D. J. & Lemontt, J. E. Function and autoregulation of yeast copperthionein. Science 228, 685–690 (1985)
Ganot, P., Bortolin, M. L. & Kiss, T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell 89, 799–809 (1997)
Ni, J., Tien, A. L. & Fournier, M. J. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell 89, 565–573 (1997)
Zhao, X. & Yu, Y. T. Detection and quantitation of RNA base modifications. RNA 10, 996–1002 (2004)
Leeds, P., Peltz, S. W., Jacobson, A. & Culbertson, M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 5, 2303–2314 (1991)
Leeds, P., Wood, J. M., Lee, B. S. & Culbertson, M. R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol. Cell. Biol. 12, 2165–2177 (1992)
Wilusz, C. J., Wang, W. & Peltz, S. W. Curbing the nonsense: the activation and regulation of mRNA surveillance. Genes Dev. 15, 1781–1785 (2001)
Agris, P. F. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog. Nucleic Acid Res. Mol. Biol. 53, 79–129 (1996)
Davis, D. R. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 23, 5020–5026 (1995)
Auffinger, P. & Westhof, E. RNA hydration: three nanoseconds of multiple molecular dynamics simulations of the solvated tRNAAsp anticodon hairpin. J. Mol. Biol. 269, 326–341 (1997)
Agris, P. F. Decoding the genome: a modified view. Nucleic Acids Res. 32, 223–238 (2004)
Song, H. et al. The crystal structure of human eukaryotic release factor eRF1—mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell 100, 311–321 (2000)
Atkins, J. F., Gesteland, R. F., Reid, B. R. & Anderson, C. W. Normal tRNAs promote ribosomal frameshifting. Cell 18, 1119–1131 (1979)
Frischmeyer, P. A. & Dietz, H. C. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 8, 1893–1900 (1999)
Wu, G., Xiao, M., Yang, C. & Yu, Y. T. U2 snRNA is inducibly pseudouridylated at novel sites by Pus7p and snR81 RNP. EMBO J. 30, 79–89 (2010)
Ma, X. et al. Pseudouridylation of yeast U2 snRNA is catalyzed by either an RNA-guided or RNA-independent mechanism. EMBO J. 24, 2403–2413 (2005)
Chernyakov, I., Whipple, J. M., Kotelawala, L., Grayhack, E. J. & Phizicky, E. M. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 22, 1369–1380 (2008)
Yu, Y. T. Site-specific 4-thiouridine incorporation into RNA molecules. Methods Enzymol. 318, 71–88 (2000)
Zhao, X. & Yu, Y. T. Pseudouridines in and near the branch site recognition region of U2 snRNA are required for snRNP biogenesis and pre-mRNA splicing in Xenopus oocytes. RNA 10, 681–690 (2004)
Gelperin, D. M. et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 19, 2816–2826 (2005)
Zebarjadian, Y., King, T., Fournier, M. J., Clarke, L. & Carbon, J. Point mutations in yeast CBF5 can abolish in vivo pseudouridylation of rRNA. Mol. Cell. Biol. 19, 7461–7472 (1999)
Acknowledgements
We thank F. Hagen and the Proteomics Core at the University of Rochester for performing the mass spectrometry analysis. We also thank E. Phizicky and B. Grayhack for the wild-type TRM4 construct, C. Guthrie for the cup1Δ yeast strain, D. Mcpheeters for the wild-type CUP1 construct, and M. Dumont for the anti-Eno1p antibody. Lastly, we would like to thank members of the Yu laboratory, especially X. Zhao, for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.K. and Y.-T.Y. designed and interpreted the experiments. Mass spectrometry was performed at the Proteomics Core at the University of Rochester Medical Center. J.K. performed all other experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-10 with legends. (PDF 1570 kb)
Rights and permissions
About this article
Cite this article
Karijolich, J., Yu, YT. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 474, 395–398 (2011). https://doi.org/10.1038/nature10165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10165
This article is cited by
-
RNA modification-mediated mRNA translation regulation in liver cancer: mechanisms and clinical perspectives
Nature Reviews Gastroenterology & Hepatology (2024)
-
N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting
Nature (2024)
-
Near-cognate tRNAs increase the efficiency and precision of pseudouridine-mediated readthrough of premature termination codons
Nature Biotechnology (2024)
-
Coordination of RNA modifications in the brain and beyond
Molecular Psychiatry (2023)
-
Quantitative sequencing using BID-seq uncovers abundant pseudouridines in mammalian mRNA at base resolution
Nature Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.