Abstract
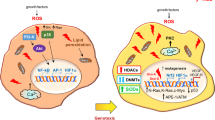
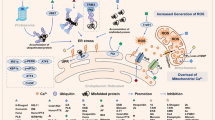
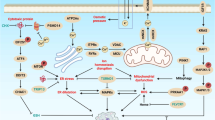
Malignant transformation, driven by gain-of-function mutations in oncogenes and loss-of-function mutations in tumour suppressor genes, results in cell deregulation that is frequently associated with enhanced cellular stress (for example, oxidative, replicative, metabolic and proteotoxic stress, and DNA damage)1. Adaptation to this stress phenotype is required for cancer cells to survive, and consequently cancer cells may become dependent upon non-oncogenes that do not ordinarily perform such a vital function in normal cells. Thus, targeting these non-oncogene dependencies in the context of a transformed genotype may result in a synthetic lethal interaction and the selective death of cancer cells2. Here we used a cell-based small-molecule screening and quantitative proteomics approach that resulted in the unbiased identification of a small molecule that selectively kills cancer cells but not normal cells. Piperlongumine increases the level of reactive oxygen species (ROS) and apoptotic cell death in both cancer cells and normal cells engineered to have a cancer genotype, irrespective of p53 status, but it has little effect on either rapidly or slowly dividing primary normal cells. Significant antitumour effects are observed in piperlongumine-treated mouse xenograft tumour models, with no apparent toxicity in normal mice. Moreover, piperlongumine potently inhibits the growth of spontaneously formed malignant breast tumours and their associated metastases in mice. Our results demonstrate the ability of a small molecule to induce apoptosis selectively in cells that have a cancer genotype, by targeting a non-oncogene co-dependency acquired through the expression of the cancer genotype in response to transformation-induced oxidative stress3,4,5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
25 July 2018
This Article has been retracted; see accompanying Retraction.
References
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009)
Yap, T. A., Sandhu, S. K., Carden, C. P. & de Bono, J. S. Poly(ADP-ribose) polymerase (PARP) inhibitors: Exploiting a synthetic lethal strategy in the clinic. CA Cancer J. Clin. 61, 31–49 (2011)
Poole, L. B. & Nelson, K. J. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 12, 18–24 (2008)
Schumacker, P. T. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10, 175–176 (2006)
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Natl Rev. 8, 579–591 (2009)
Brown, L. et al. CDIP, a novel pro-apoptotic gene, regulates TNFα-mediated apoptosis in a p53-dependent manner. EMBO J. 26, 3410–3422 (2007)
Bezerra, D. P. et al. Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol. In Vitro 21, 1–8 (2007)
Hahn, W. C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999)
Ryo, A. et al. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 22, 5281–5295 (2002)
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12, 954–961 (1992)
Ong, S. E. et al. Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc. Natl Acad. Sci. USA 106, 4617–4622 (2009)
Bateman, R. L., Rauh, D., Tavshanjian, B. & Shokat, K. M. Human carbonyl reductase 1 is an S-nitrosoglutathione reductase. J. Biol. Chem. 283, 35756–35762 (2008)
Ralat, L. A., Manevich, Y., Fisher, A. B. & Colman, R. F. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase π with activity changes in both enzymes. Biochemistry 45, 360–372 (2006)
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009)
Fruehauf, J. P. & Meyskens, F. L., Jr Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 13, 789–794 (2007)
Huang, P., Feng, L., Oldham, E. A., Keating, M. J. & Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407, 390–395 (2000)
Trachootham, D. et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10, 241–252 (2006)
Gogvadze, V., Orrenius, S. & Zhivotovsky, B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 18, 165–173 (2008)
Szatrowski, T. P. & Nathan, C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51, 794–798 (1991)
Ravindran, J., Prasad, S. & Aggarwal, B. B. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? Am. Assoc. Pharm. Sci. J. 11, 495–510 (2009)
Yue, P., Zhou, Z., Khuri, F. R. & Sun, S. Y. Depletion of intracellular glutathione contributes to JNK-mediated death receptor 5 upregulation and apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me). Cancer Biol. Ther. 5, 492–497 (2006)
Banning, A., Deubel, S., Kluth, D., Zhou, Z. & Brigelius-Flohe, R. The GI-GPx gene is a target for Nrf2. Mol. Cell Biol. 25, 4914–4923 (2005)
Dinkova-Kostova, A. T. et al. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with KEAP1. J. Biol. Chem. 285, 33747–33755 (2010)
Lee, A. C. et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274, 7936–7940 (1999)
Schramek, D. et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nature Genet. 43, 212–219 (2011)
Wang, T., Arifoglu, P., Ronai, Z. & Tew, K. D. Glutathione S-transferase P1–1 (GSTP1–1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 276, 20999–21003 (2001)
Margolin, A. A. et al. Empirical Bayes analysis of quantitative proteomics experiments. PLoS ONE 4, e7454 (2009)
Acknowledgements
We thank K. Chu, L. Brown-Endres, E. Lerner and F. Neville for their help in preparing the manuscript, W. C. Hahn for BJ cell lines, V. Band for 76N cells, D. Beer for H1975 cells and K. Todorova, G. Wei, S. Ong, S. Norton and F. An for technical assistance. This project has been supported in part by grants CA142805, CA127247, CA085681 and CA080058 from NIH. This research was supported by the National Cancer Institute’s Initiative for Chemical Genetics Contract (N01-CO-12400) and Cancer Target Discovery and Development Network grant (5 RC2 CA148399-02), as well as the National Institutes of Health Genomics Based Drug Discovery—Target ID Project Grant (RL1HG004671, which is administratively linked to National Institutes of Health Grants RL1CA133834, RL1GM084437 and UL1RR024924). S.L.S. is an Investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
L.R. and T.I. conducted most of the experimental work. A.U.G., M.S. and X.L. made critical experimental contributions. N.J.T., A.M.S., T.R.G., S.A.C., A.F.S. and M.F. designed the experimental plans, analysed and interpreted the data. A.M., S.L.S. and S.W.L. designed and directed the project and drafted the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.W.L., A.M. and M.F. are co-founders and consultants of Canthera Therapeutics Inc., which pursues cancer therapeutics that act through ROS mechanisms.
Supplementary information
Supplementary Information
The file contains Supplementary Methods, Supplementary Tables 1-2, Supplementary Figures 1-33 with legends and additional references. (PDF 7962 kb)
Rights and permissions
About this article
Cite this article
Raj, L., Ide, T., Gurkar, A. et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475, 231–234 (2011). https://doi.org/10.1038/nature10167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10167
This article is cited by
-
Comparative assessment of direct and indirect cold atmospheric plasma effects, based on helium and argon, on human glioblastoma: an in vitro and in vivo study
Scientific Reports (2024)
-
Cysteine depletion sensitizes prostate cancer cells to agents that enhance DNA damage and to immune checkpoint inhibition
Journal of Experimental & Clinical Cancer Research (2023)
-
Targeting NRF2 uncovered an intrinsic susceptibility of acute myeloid leukemia cells to ferroptosis
Experimental Hematology & Oncology (2023)
-
In vivo metallophilic self-assembly of a light-activated anticancer drug
Nature Chemistry (2023)
-
Preparation of rutin-loaded mesoporous silica nanoparticles and evaluation of its physicochemical, anticancer, and antibacterial properties
Molecular Biology Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.