Abstract
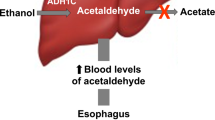
Reactive aldehydes are common carcinogens. They are also by-products of several metabolic pathways and, without enzymatic catabolism, may accumulate and cause DNA damage. Ethanol, which is metabolised to acetaldehyde, is both carcinogenic and teratogenic in humans. Here we find that the Fanconi anaemia DNA repair pathway counteracts acetaldehyde-induced genotoxicity in mice. Our results show that the acetaldehyde-catabolising enzyme Aldh2 is essential for the development of Fancd2−/− embryos. Nevertheless, acetaldehyde-catabolism-competent mothers (Aldh2+/− ) can support the development of double-mutant (Aldh2−/−Fancd2−/− ) mice. However, these embryos are unusually sensitive to ethanol exposure in utero, and ethanol consumption by postnatal double-deficient mice rapidly precipitates bone marrow failure. Lastly, Aldh2−/−Fancd2−/− mice spontaneously develop acute leukaemia. Acetaldehyde-mediated DNA damage may critically contribute to the genesis of fetal alcohol syndrome in fetuses, as well as to abnormal development, haematopoietic failure and cancer predisposition in Fanconi anaemia patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362, 709–715 (1993)
Patel, K. J. & Joenje, H. Fanconi anemia and DNA replication repair. DNA Repair (Amst.) 6, 885–890 (2007)
O’Brien, P. J., Siraki, A. G. & Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 35, 609–662 (2005)
Wang, M. et al. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 13, 1149–1157 (2000)
Stein, S., Lao, Y., Yang, I. Y., Hecht, S. S. & Moriya, M. Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1,N2-propanodeoxyguanosine DNA adducts in human cells. Mutat. Res. 608, 1–7 (2006)
Cheng, G. et al. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem. Res. Toxicol. 16, 145–152 (2003)
Vasiliou, V., Pappa, A. & Estey, T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab. Rev. 36, 279–299 (2004)
Perez-Miller, S. et al. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nature Struct. Mol. Biol. 17, 159–164 (2010)
Ridpath, J. R. et al. Cells deficient in the FANC/BRCA pathway are hypersensitive to plasma levels of formaldehyde. Cancer Res. 67, 11117–11122 (2007)
Nagayoshi, H. et al. Increased formation of gastric N2-ethylidene-2′-deoxyguanosine DNA adducts in aldehyde dehydrogenase-2 knockout mice treated with ethanol. Mutat. Res. 673, 74–77 (2009)
Matsuda, T. et al. Increased formation of hepatic N2-ethylidene-2′-deoxyguanosine DNA adducts in aldehyde dehydrogenase-2 knockout mice treated with ethanol. Carcinogenesis 28, 2363–2366 (2007)
Seitz, H. K. & Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Rev. Cancer 7, 599–612 (2007)
Chen, L. et al. Quantitation of an acetaldehyde adduct in human leukocyte DNA and the effect of smoking cessation. Chem. Res. Toxicol. 20, 108–113 (2007)
Niedzwiedz, W. et al. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol. Cell 15, 607–620 (2004)
Alpi, A. et al. UBE2T, the Fanconi anemia core complex, and FANCD2 are recruited independently to chromatin: a basis for the regulation of FANCD2 monoubiquitination. Mol. Cell. Biol. 27, 8421–8430 (2007)
King, G. & Holmes, R. Human ocular aldehyde dehydrogenase isozymes: distribution and properties as major soluble proteins in cornea and lens. J. Exp. Zool. 282, 12–17 (1998)
Pappa, A., Estey, T., Manzer, R., Brown, D. & Vasiliou, V. Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem. J. 376, 615–623 (2003)
Riveros-Rosas, H., Julian-Sanchez, A. & Pina, E. Enzymology of ethanol and acetaldehyde metabolism in mammals. Arch. Med. Res. 28, 453–471 (1997)
Kunitoh, S. et al. Acetaldehyde as well as ethanol is metabolized by human CYP2E1. J. Pharmacol. Exp. Ther. 280, 527–532 (1997)
Parmar, K., D’Andrea, A. & Niedernhofer, L. J. Mouse models of Fanconi anemia. Mutat. Res. 668, 133–140 (2009)
Crossan, G. P. et al. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nature Genet. 43, 147–152 (2011)
Marietta, C., Thompson, L. H., Lamerdin, J. E. & Brooks, P. J. Acetaldehyde stimulates FANCD2 monoubiquitination, H2AX phosphorylation, and BRCA1 phosphorylation in human cells in vitro: implications for alcohol-related carcinogenesis. Mutat. Res. 664, 77–83 (2009)
Yu, H. S. et al. Characteristics of aldehyde dehydrogenase 2 (Aldh2) knockout mice. Toxicol. Mech. Methods 19, 535–540 (2009)
Houghtaling, S. et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 17, 2021–2035 (2003)
Sulik, K. K., Johnston, M. C. & Webb, M. A. Fetal alcohol syndrome: embryogenesis in a mouse model. Science 214, 936–938 (1981)
Webster, W. S., Walsh, D. A., McEwen, S. E. & Lipson, A. H. Some teratogenic properties of ethanol and acetaldehyde in C57BL/6J mice: implications for the study of the fetal alcohol syndrome. Teratology 27, 231–243 (1983)
O’Shea, K. S. & Kaufman, M. H. The teratogenic effect of acetaldehyde: implications for the study of the fetal alcohol syndrome. J. Anat. 128, 65–76 (1979)
Michot, F. & Gut, J. Alcohol-induced bone marrow damage. A bone marrow study in alcohol-dependent individuals. Acta Haematol. 78, 252–257 (1987)
Nakao, S., Harada, M., Kondo, K., Mizushima, N. & Matsuda, T. Reversible bone marrow hypoplasia induced by alcohol. Am. J. Hematol. 37, 120–123 (1991)
Meagher, R. C., Sieber, F. & Spivak, J. L. Suppression of hematopoietic-progenitor-cell proliferation by ethanol and acetaldehyde. N. Engl. J. Med. 307, 845–849 (1982)
Marc, N., Fautrel, A., Damon, M., Guillouzo, A. & Corcos, L. Phenobarbital induction of aldehyde dehydrogenase type 2 mRNA in mouse liver: a candidate region on chromosome 7 for a putative regulatory gene. Biochem. Genet. 38, 297–308 (2000)
Vasiliou, V., Torronen, R., Malamas, M. & Marselos, M. Inducibility of liver cytosolic aldehyde dehydrogenase activity in various animal species. Comp. Biochem. Physiol. C 94, 671–675 (1989)
Chen, C.-H. et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 321, 1493–1495 (2008)
Perez-Miller, S. et al. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nature Struct. Mol. Biol. 17, 159–164 (2010)
Knipscheer, P. et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science 326, 1698–1701 (2009)
Abel, E. L. & Sokol, R. J. A revised conservative estimate of the incidence of FAS and its economic impact. Alcohol. Clin. Exp. Res. 15, 514–524 (1991)
Latino-Martel, P. et al. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 19, 1238–1260 (2010)
MacArthur, A. C. et al. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes Control 19, 283–295 (2008)
Brooks, P. J., Enoch, M. A., Goldman, D., Li, T. K. & Yokoyama, A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 6, e50 (2009)
McKay, J. D. et al. A genome-wide association study of upper aerodigestive tract cancers conducted within the INHANCE Consortium. PLoS Genet. 7, e1001333 (2011)
Alter, B. P., Joenje, H., Oostra, A. B. & Pals, G. Fanconi anemia: adult head and neck cancer and hematopoietic mosaicism. Arch. Otolaryngol. Head Neck Surg. 131, 635–639 (2005)
Hazen, S. L., Hsu, F. F., d’Avignon, A. & Heinecke, J. W. Human neutrophils employ myeloperoxidase to convert α-amino acids to a battery of reactive aldehydes: a pathway for aldehyde generation at sites of inflammation. Biochemistry 37, 6864–6873 (1998)
O’Brien, P. J., Siraki, A. G. & Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 35, 609–662 (2005)
Acknowledgements
We thank M. Grompe for Fancd2 knockout mice, J. Sale and S. Takeda for DT40 strains, N. Sugimura and F. Gergely for comments on the manuscript. We are grateful to T. Langford, R. Berks, V. Smith, J. Wiles, C. Shepherd, M. Kidd, M. Brown, A. Mead, R. Pannell, J. Garaycoechea and A. Shortland for their assistance with animal experiments and husbandry. We thank N. Grant and P. Banks from the Visual Aids department for photographic images and W. Zhao of the Human Research Tissue Bank (NIHR Cambridge Biomedical Research Centre) for processing histology. F.L. and I.V.R. are funded by the Children’s Leukaemia Trust and Fanconi Anaemia Research Fund, respectively. K.J.P. acknowledges M. Neuberger, N. McIntyre and C. Desai for support.
Author information
Authors and Affiliations
Contributions
K.J.P., F.L. and G.P.C. designed the experiments. F.L. and G.P.C. performed the majority of the experimental work, I.V.R. contributed to DT40 clonogenic assays and FACS analysis of tumours. M.J.A. carried out histological analysis. K.J.P. wrote the manuscript assisted by F.L. and G.P.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-8 with legends. (PDF 2408 kb)
Rights and permissions
About this article
Cite this article
Langevin, F., Crossan, G., Rosado, I. et al. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 475, 53–58 (2011). https://doi.org/10.1038/nature10192
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10192
This article is cited by
-
FLIP(C1orf112)-FIGNL1 complex regulates RAD51 chromatin association to promote viability after replication stress
Nature Communications (2024)
-
Hypomorphic Brca2 and Rad51c double mutant mice display Fanconi anemia, cancer and polygenic replication stress
Nature Communications (2023)
-
A CRISPR-Cas9 screen identifies EXO1 as a formaldehyde resistance gene
Nature Communications (2023)
-
A common East-Asian ALDH2 mutation causes metabolic disorders and the therapeutic effect of ALDH2 activators
Nature Communications (2023)
-
Effects of the major formaldehyde catalyzer ADH5 on phenotypes of fanconi anemia zebrafish model
Molecular Biology Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.