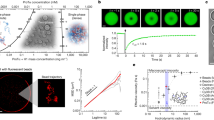
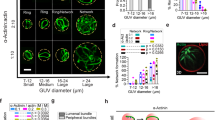
Abstract
Cells are organized on length scales ranging from ångström to micrometres. However, the mechanisms by which ångström-scale molecular properties are translated to micrometre-scale macroscopic properties are not well understood. Here we show that interactions between diverse synthetic, multivalent macromolecules (including multi-domain proteins and RNA) produce sharp liquid–liquid-demixing phase separations, generating micrometre-sized liquid droplets in aqueous solution. This macroscopic transition corresponds to a molecular transition between small complexes and large, dynamic supramolecular polymers. The concentrations needed for phase transition are directly related to the valency of the interacting species. In the case of the actin-regulatory protein called neural Wiskott–Aldrich syndrome protein (N-WASP) interacting with its established biological partners NCK and phosphorylated nephrin1, the phase transition corresponds to a sharp increase in activity towards an actin nucleation factor, the Arp2/3 complex. The transition is governed by the degree of phosphorylation of nephrin, explaining how this property of the system can be controlled to regulatory effect by kinases. The widespread occurrence of multivalent systems suggests that phase transitions may be used to spatially organize and biochemically regulate information throughout biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jones, N. et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 (2006)
Flory, P. J. Principles of Polymer Chemistry (Cornell Univ. Press, 1953)
Cohen, R. J. & Benedek, G. B. Equilibrium and kinetic theory of polymerization and the sol-gel transition. J. Phys. Chem. 86, 3696–3714 (1982)
Lehn, J.-M. Supramolecular polymer chemistry—scope and perspectives. Polym. Int. 51, 825–839 (2002)
Tanaka, F. Polymer Physics: Applications to Molecular Association and Thermoreversible Gelation (Cambridge Univ. Press, 2011)
Semenov, A. N. & Rubinstein, M. Thermoreversible gelation in solutions of associative polymers. 1. Statics. Macromolecules 31, 1373–1385 (1998)
Brewer, C. F., Miceli, M. C. & Baum, L. G. Clusters, bundles, arrays and lattices: novel mechanisms for lectin–saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 12, 616–623 (2002)
Pawson, T. & Nash, P. Assembly of cell regulatory systems through protein interaction domains. Science 300, 445–452 (2003)
Lunde, B. M., Moore, C. & Varani, G. RNA-binding proteins: modular design for efficient function. Nature Rev. Mol. Cell Biol. 8, 479–490 (2007)
Ruthenburg, A. J., Li, H., Patel, D. J. & Allis, C. D. Multivalent engagement of chromatin modifications by linked binding modules. Nature Rev. Mol. Cell Biol. 8, 983–994 (2007)
Goldberg, R. A theory of antibody–antigen reactions. I. Theory for reactions of multivalent antigen with bivalent and univalent antibody. J. Am. Chem. Soc. 74, 5715–5725 (1952)
Dam, T. K. et al. Thermodynamic, kinetic, and electron microscopy studies of concanavalin A and Dioclea grandiflora lectin cross-linked with synthetic divalent carbohydrates. J. Biol. Chem. 280, 8640–8646 (2005)
Sisu, C. et al. The influence of ligand valency on aggregation mechanisms for inhibiting bacterial toxins. Chembiochem 10, 329–337 (2009)
Jin, J. et al. Eukaryotic protein domains as functional units of cellular evolution. Sci. Signal. 2, ra76 (2009)
Asherie, N. et al. Oligomerization and phase separation in globular protein solutions. Biophys. Chem. 75, 213–227 (1998)
Stockmayer, W. H. Molecular distribution in condensation polymers. J. Polym. Sci. 9, 69–71 (1952)
Li, J., Ngai, T. & Wu, C. The slow relaxation mode: from solutions to gel networks. Polym. J. 42, 609–625 (2010)
Semenov, A., Charlot, A., Auzely-Velty, R. & Rinaudo, M. Rheological properties of binary associating polymers. Rheol. Acta 46, 541–568 (2007)
Blasutig, I. M. et al. Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol. Cell. Biol. 28, 2035–2046 (2008)
Rohatgi, R., Nollau, P., Ho, H. Y., Kirschner, M. W. & Mayer, B. J. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP–Arp2/3 pathway. J. Biol. Chem. 276, 26448–26452 (2001)
Padrick, S. B. et al. Hierarchical regulation of WASP/WAVE proteins. Mol. Cell 32, 426–438 (2008)
Padrick, S. B. & Rosen, M. K. Physical mechanisms of signal integration by WASP family proteins. Annu. Rev. Biochem. 79, 707–735 (2010)
Lettau, M., Pieper, J. & Janssen, O. Nck adaptor proteins: functional versatility in T cells. Cell Commun. Signal. 7, 1 (2009)
Obenauer, J. C., Cantley, L. C. & Yaffe, M. B. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 31, 3635–3641 (2003)
Matera, A. G., Izaguire-Sierra, M., Praveen, K. & Rajendra, T. K. Nuclear bodies: random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev. Cell 17, 639–647 (2009)
Buchan, J. R. & Parker, R. Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941 (2009)
Parker, R. & Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 (2007)
Matera, A. G. & Shpargel, K. B. Pumping RNA: nuclear bodybuilding along the RNP pipeline. Curr. Opin. Cell Biol. 18, 317–324 (2006)
Bernardi, R. & Pandolfi, P. P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nature Rev. Mol. Cell Biol. 8, 1006–1016 (2007)
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009)
Chen, B. et al. ATP ground- and transition states of bacterial enhancer binding AAA+ ATPases support complex formation with their target protein, σ54. Structure 15, 429–440 (2007)
Svergun, D. I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 (1992)
Fischer, H., Neto, M. O., Napolitano, H. B., Polikarpov, I. & Craievich, A. F. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J. Appl. Crystallogr. 43, 101–109 (2010)
Provencher, S. W. CONTIN: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 27, 229–242 (1982)
Acknowledgements
We thank J. Onuchic and S. Padrick for discussion of the theoretical aspects of this study, L. Rice for sharing his fluorescence microscope, M. Socolich for a gift of purified eGFP, K. Luby-Phelps and A. Bugde for advice on FRAP experiments, S. Padrick and L. Doolittle for help in purifying actin and the Arp2/3 complex and for sharing reagents, N. Grishin and S. Shi for help with database searches, K. Lynch for providing the PTB expression construct, D. Billadeau and T. Gomez for providing antibodies, A. Ramesh, W. Winkler and P.-L. Tsai for advice on RNA experiments, K. Roybal and C. Wülfing for sharing unpublished data, and J. Liu for help with cryo-electron tomography. This work was supported by the following: the Howard Hughes Medical Institute and grants from the National Institutes of Health (NIH) (R01-GM56322) and Welch Foundation (I–1544) to M.K.R., a Chilton Foundation Fellowship to H.-C.C., an NIH EUREKA award (R01-GM088745) to Q.-X.J., an NIH Cancer Biology T32 Training Grant to M.L., a National Science Foundation award (DMR-1005707) to P.S.R. and a Gates Millennium Fund award to J.V.H. Use of the Advanced Photon Source was supported by the US Department of Energy, Basic Energy Sciences, Office of Science, under contract number W-31-109-ENG-38. BioCAT is NIH-supported Research Center RR-08630.
Author information
Authors and Affiliations
Contributions
M.K.R. oversaw the project, helped analyse all of the data and wrote the paper with assistance from all of the authors. P.L., H.-.C.C. and M.K.R. conceived of the project. P.L. developed and interpreted the theoretical and computational models, which promoted much of the experimentation. S.B. performed and analysed experiments on the nephrin–NCK–N-WASP system and performed monovalent competition studies. H.-.C.C. mapped and analysed the phase diagrams, and collected FRAP data, on the engineered model systems. S.K. performed and analysed the cellular experiments. S.B., B.C., L.G. and B.T.N. collected and/or analysed the SAXS data. S.B., M.L. and Q.-.X. J. collected and/or analysed the electron microscopy data. S.B., J.V.H. and P.S.R. collected and/or analysed the multi-angle DLS data. H.-.C.C. and S.B. collected and analysed the single-angle DLS data. D.S.K. synthesized the octameric PRM dendrimer. S.F.B. analysed the cyclization in the sol–gel transition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Table 1, full legend for Supplementary Movie 1, Supplementary References and Supplementary Figures 1-23. (PDF 24828 kb)
Supplementary Movie 1
This zipped file contains a movie showing large polymer formation – see Supplementary Information file page 23 for full legend. (ZIP 218 kb)
Rights and permissions
About this article
Cite this article
Li, P., Banjade, S., Cheng, HC. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012). https://doi.org/10.1038/nature10879
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10879
This article is cited by
-
Crosstalk between protein post-translational modifications and phase separation
Cell Communication and Signaling (2024)
-
Physiology and pharmacological targeting of phase separation
Journal of Biomedical Science (2024)
-
Kinetic control of shape deformations and membrane phase separation inside giant vesicles
Nature Chemistry (2024)
-
A chaperone-like function of FUS ensures TAZ condensate dynamics and transcriptional activation
Nature Cell Biology (2024)
-
Higher-order protein assembly controls kinetochore formation
Nature Cell Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.