Abstract
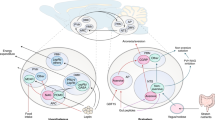
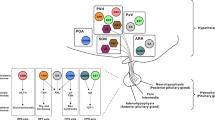
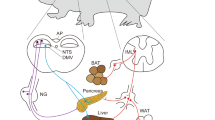
Although it is a widely held thought that direct hormone action on peripheral tissues is sufficient to mediate the control of nutrient handling, the role of the central nervous system in certain aspects of metabolism has long been recognized. Furthermore, recent findings have suggested a more general role for the central nervous system in metabolic control, and have revealed the importance of a number of cues and hypothalamic circuits. The brain's contributions to metabolic control are more readily revealed and play a crucial part in catabolic states or in hormone deficiencies that mimic starvation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Levin, B. E. Neuronal glucose sensing: still a physiological orphan? Cell Metab. 6, 252–254 (2007).
Levin, B. E. & Sherwin, R. S. Peripheral glucose homeostasis: does brain insulin matter? J. Clin. Invest. 121, 3392–3395 (2011).
Pocai, A., Obici, S., Schwartz, G. J. & Rossetti, L. A brain–liver circuit regulates glucose homeostasis. Cell Metab. 1, 53–61 (2005).
Taborsky, G. J. Jr. Islets have a lot of nerve! Or do they? Cell Metab. 14, 5–6 (2011).
Wiesli, P. et al. Acute psychological stress affects glucose concentrations in patients with type 1 diabetes following food intake but not in the fasting state. Diabetes Care 28, 1910–1915 (2005).
McCowen, K. C., Malhotra, A. & Bistrian, B. R. Stress-induced hyperglycemia. Crit. Care Clin. 17, 107–124 (2001).
Levin, B. E., Routh, V. H., Kang, L., Sanders, N. M. & Dunn-Meynell, A. A. Neuronal glucosensing: what do we know after 50 years? Diabetes 53, 2521–2528 (2004)
Evans, M. L. & Sherwin, R. S. Blood glucose and the brain in diabetes: between a rock and a hard place? Curr. Diab. Rep. 2, 101–102 (2002).
Ritter, S., Li, A. J., Wang, Q. & Dinh, T. T. The value of looking backward: the essential role of the hindbrain in counter regulatory responses to glucose deficit. Endocrinology 152, 4019–4032 (2011).
Schwartz, M. W. & Porte, D. Jr. Diabetes, obesity, and the brain. Science 307, 375–379 (2005).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Rossetti, L. et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J. Biol. Chem. 272, 27758–27763 (1997).
Liu, L. et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J. Biol. Chem. 273, 31160–31167 (1998).
Obici, S., Zhang, B. B., Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature Med. 8, 1376–1382 (2002).
Gutierrez-Juarez, R., Obici, S. & Rossetti, L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J. Biol. Chem. 279, 49704–49715 (2004).
Pocai, A. et al. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54, 3182–3189 (2005).
Ahima, R. S. Central actions of adipocyte hormones. Trends Endocrinol. Metab. 16, 307–313 (2005).
D'Alessio, D. A., Sandoval, D. A. & Seeley, R. J. New ways in which GLP-1 can regulate glucose homeostasis. J. Clin. Invest. 115, 3406–3408 (2005).
Briggs, D. I. & Andrews, Z. B. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology 93, 48–57 (2011).
Heppner, K. M., Tong, J., Kirchner, H., Nass, R. & Tschop, M. H. The ghrelin O-acyltransferase-ghrelin system: a novel regulator of glucose metabolism. Curr. Opin. Endocrinol. Diabetes Obes. 18, 50–55 (2011).
Kleinridders, A., Konner, A. C. & Bruning, J. C. CNS-targets in control of energy and glucose homeostasis. Curr. Opin. Pharmacol. 9, 794–804 (2009).
Hribal, M. L., Oriente, F. & Accili, D. Mouse models of insulin resistance. Am. J. Physiol. Endocrinol. Metab. 282, E977–E981 (2002).
Williams, K. W., Scott, M. M. & Elmquist, J. K. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. Eur. J. Pharmacol. 660, 2–12 (2011).
Minokoshi, Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574 (2004).
Pocai, A., Muse, E. D. & Rossetti, L. Did a muscle fuel gauge conquer the brain? Nature Med. 12, 50–51 (2006).
Cota, D. et al. Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 (2006).
Diano, S. & Horvath, T. L. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol. Med. 18, 52–58 (2012).
Lam, T. K. et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nature Med. 11, 320–327 (2005).
Vanpatten, S., Karkanias, G. B., Rossetti, L. & Cohen, D. E. Intracerebroventricular leptin regulates hepatic cholesterol metabolism. Biochem. J. 379, 229–233 (2004).
Nogueiras, R. et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 117, 3475–3488 (2007). Using pharmacological and genetic approaches in rodents, this article demonstrates a role for endogenous CNS melanocortin action in the control of whole-body lipid metabolism.
Burdakov, D. & Alexopoulos, H. Metabolic state signalling through central hypocretin/orexin neurons. J. Cell. Mol. Med. 9, 795–803 (2005).
Myers, M. G. Jr, Munzberg, H., Leinninger, G. M. & Leshan, R. L. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 9, 117–123 (2009). This review highlights the idea that most LRb neurons in the brain are distinct from the canonical pro-opiomelanocortin and AgRP neurons and lie outside the arcuate nucleus.
Xu, Y., Elmquist, J. K. & Fukuda, M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann. NY Acad. Sci. 1243, 1–14 (2011).
Sadagurski, M. et al. IRS2 signaling in LepR-b neurons suppresses Foxo1 to control energy balance independently of leptin action. Cell Metab. 15, 703–712 (2012).
Taguchi, A., Wartschow, L. M. & White, M. F. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317, 369–372 (2007).
Patterson, C. M. et al. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol. Metab. http://dx.doi.org/10.1016/j.molmet.2012.05.001 (25 July 2012).
Bates, S. H. et al. STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003).
Buettner, C. et al. Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 4, 49–60 (2006).
Robertson, S. et al. Insufficiency of Janus kinase 2-autonomous leptin receptor signals for most physiologic leptin actions. Diabetes 59, 782–790 (2010).
Jiang, L. et al. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc. Natl Acad. Sci. USA 105, 18619–18624 (2008).
Barnes, M. B., Lawson, M. A. & Beverly, J. L. Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1815–R1820 (2011).
Levin, B. E., Magnan, C., Dunn-Meynell, A. & Le Foll, C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152, 2552–2557 (2011).
Chan, O., Lawson, M., Zhu, W., Beverly, J. L. & Sherwin, R. S. ATP-sensitive K+ channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 56, 1120–1126 (2007).
Kang, L. et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55, 412–420 (2006).
Elias, C. F. et al. Chemical characterization of leptin-activated neurons in the rat brain. J. Comp. Neurol. 423, 261–281 (2000).
Haque, M. S. et al. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48, 1706–1712 (1999).
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Bingham, N. C., Anderson, K. K., Reuter, A. L., Stallings, N. R. & Parker, K. L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149, 2138–2148 (2008).
Klockener, T. et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nature Neurosci. 14, 911–918 (2011).
Kim, K. W. et al. FOXO1 in the ventromedial hypothalamus regulates energy balance. J. Clin. Invest. 122, 2578–2589 (2012).
Bergen, H. T., Mizuno, T. M., Taylor, J. & Mobbs, C. V. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology 139, 4483–4488 (1998).
Coppari, R. et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 1, 63–72 (2005). This article reports the reactivation of LRb specifically in the arcuate nucleus; although this only slightly altered food intake and adiposity, glucose homeostasis was markedly improved, suggesting an important role for arcuate-nucleus leptin action in the control of glucose homeostasis independently of energy balance.
Morton, G. J. et al. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fak/fak) rats. Endocrinology 144, 2016–2024 (2003).
Morton, G. J. et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2, 411–420 (2005). Using molecular–viral and pharmacological approaches, the authors show that insulin-dependent signalling pathways in the arcuate nucleus are crucial for the control of glucose homeostasis by leptin.
Schwartz, M. W. Central nervous system regulation of food intake. Obesity (Silver Spring) 14, 1S–8S (2006).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Hill, J. W. et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 11, 286–297 (2010).
Lin, H. V. et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59, 337–346 (2010).
Padilla, S. L., Carmody, J. S. & Zeltser, L. M. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Med. 16, 403–405 (2010).
Berglund, E. D. et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Invest. 122, 1000–1009 (2012).
Huo, L. et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 9, 537–547 (2009).
Hill, J. W. et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 150, 4874–4882 (2009).
Perez-Tilve, D. et al. Melanocortin signaling in the CNS directly regulates circulating cholesterol. Nature Neurosci. 13, 877–882 (2010).
Shrestha, Y. B. et al. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R140–R149 (2010).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Ste, M. L., Miura, G. I., Marsh, D. J., Yagaloff, K. & Palmiter, R. D. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc. Natl Acad. Sci. USA 97, 12339–12344 (2000).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Sutton, G. M. et al. Diet–genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147, 2183–2196 (2006).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 26, 97–102 (2000).
Butler, A. A. The melanocortin system and energy balance. Peptides 27, 281–290 (2006).
Grill, H. J. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14, 216S–221S (2006).
de Backer, M. W. et al. Melanocortin receptor-mediated effects on obesity are distributed over specific hypothalamic regions. Int. J. Obes. (Lond.) 35, 629–641 (2011).
Cherrington, A. D. The role of hepatic insulin receptors in the regulation of glucose production. J. Clin. Invest. 115, 1136–1139 (2005).
Shimomura, I., Hammer, R. E., Ikemoto, S., Brown, M. S. & Goldstein, J. L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 (1999).
Oral, E. A. et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346, 570–578 (2002).
Wang, M. Y. et al. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl Acad. Sci. USA 107, 4813–4819 (2010).
German, J. P. et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes 59, 1626–1634 (2010). In this article, the authors show that brain leptin injection — in the absence of endogenous or exogenous insulin — suffices to normalize blood glucose.
Grill, H. J., Ginsberg, A. B., Seeley, R. J. & Kaplan, J. M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, 10128–10135 (1998).
Williams, D. L., Kaplan, J. M. & Grill, H. J. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141, 1332–1337 (2000).
Boghossian, S., Park, M. & York, D. A. Melanocortin activity in the amygdala controls appetite for dietary fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R385–R393 (2010).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
Begriche, K. et al. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes Brain Behav. 11, 291–302 (2012).
Acknowledgements
The authors thank members of the Myers and Olson laboratories for discussions and scientific insight. M.G.M. is supported by the Marilyn H. Vincent Foundation and by grants from the National Institutes of Health and the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Myers, M., Olson, D. Central nervous system control of metabolism. Nature 491, 357–363 (2012). https://doi.org/10.1038/nature11705
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11705
This article is cited by
-
Hypothalamic control of energy expenditure and thermogenesis
Experimental & Molecular Medicine (2022)
-
Brain fractalkine-CX3CR1 signalling is anti-obesity system as anorexigenic and anti-inflammatory actions in diet-induced obese mice
Scientific Reports (2022)
-
PI3K signalling at the intersection of cardio-oncology networks: cardiac safety in the era of AI
Cellular and Molecular Life Sciences (2022)
-
Stimulation of the hepatoportal nerve plexus with focused ultrasound restores glucose homoeostasis in diabetic mice, rats and swine
Nature Biomedical Engineering (2022)
-
Prostaglandin in the ventromedial hypothalamus regulates peripheral glucose metabolism
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.