Abstract
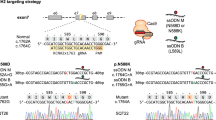
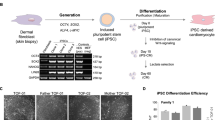
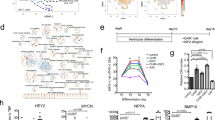
Cellular reprogramming of somatic cells to patient-specific induced pluripotent stem cells (iPSCs) enables in vitro modelling of human genetic disorders for pathogenic investigations and therapeutic screens1,2,3,4,5,6,7. However, using iPSC-derived cardiomyocytes (iPSC-CMs) to model an adult-onset heart disease remains challenging owing to the uncertainty regarding the ability of relatively immature iPSC-CMs to fully recapitulate adult disease phenotypes. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is an inherited heart disease characterized by pathological fatty infiltration and cardiomyocyte loss predominantly in the right ventricle8, which is associated with life-threatening ventricular arrhythmias. Over 50% of affected individuals have desmosome gene mutations, most commonly in PKP2, encoding plakophilin-2 (ref. 9). The median age at presentation of ARVD/C is 26 years8. We used previously published methods1,10 to generate iPSC lines from fibroblasts of two patients with ARVD/C and PKP2 mutations11,12. Mutant PKP2 iPSC-CMs demonstrate abnormal plakoglobin nuclear translocation and decreased β-catenin activity13 in cardiogenic conditions; yet, these abnormal features are insufficient to reproduce the pathological phenotypes of ARVD/C in standard cardiogenic conditions. Here we show that induction of adult-like metabolic energetics from an embryonic/glycolytic state and abnormal peroxisome proliferator-activated receptor gamma (PPAR-γ) activation underlie the pathogenesis of ARVD/C. By co-activating normal PPAR-alpha-dependent metabolism and abnormal PPAR-γ pathway in beating embryoid bodies (EBs) with defined media, we established an efficient ARVD/C in vitro model within 2 months. This model manifests exaggerated lipogenesis and apoptosis in mutant PKP2 iPSC-CMs. iPSC-CMs with a homozygous PKP2 mutation also had calcium-handling deficits. Our study is the first to demonstrate that induction of adult-like metabolism has a critical role in establishing an adult-onset disease model using patient-specific iPSCs. Using this model, we revealed crucial pathogenic insights that metabolic derangement in adult-like metabolic milieu underlies ARVD/C pathologies, enabling us to propose novel disease-modifying therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007)
Park, I. H. et al. Disease-specific induced pluripotent stem cells. Cell 134, 877–886 (2008)
Carvajal-Vergara, X. et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465, 808–812 (2010)
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010)
Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–229 (2011)
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011)
Calkins, H. & Marcus, F. Arrhythmogenic right ventricular cardiomyopathy/ dysplasia: an update. Curr. Cardiol. Rep. 10, 367–375 (2008)
Awad, M. M., Calkins, H. & Judge, D. P. Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nature Clin. Pract. Cardiovasc. Med. 5, 258–267 (2008)
Okita, K. et al. A more efficient method to generate integration-free human iPS cells. Nature Methods 8, 409–412 (2011)
Awad, M. M. et al. Recessive arrhythmogenic right ventricular dysplasia due to novel cryptic splice mutation in PKP2 . Hum. Mutat. 27, 1157 (2006)
Dalal, D. et al. Clinical features of arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in plakophilin-2. Circulation 113, 1641–1649 (2006)
Garcia-Gras, E. et al. Suppression of canonical Wnt/β-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Invest. 116, 2012–2021 (2006)
Kim, C. et al. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 19, 783–795 (2010)
Onay-Besikci, A. Regulation of cardiac energy metabolism in newborn. Mol. Cell. Biochem. 287, 1–11 (2006)
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S. & Stanley, W. C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 (2010)
Ali, A. T. et al. The relationship between alkaline phosphatase activity and intracellular lipid accumulation in murine 3T3–L1 cells and human preadipocytes. Anal. Biochem. 354, 247–254 (2006)
Taura, D. et al. Adipogenic differentiation of human induced pluripotent stem cells: comparison with that of human embryonic stem cells. FEBS Lett. 583, 1029–1033 (2009)
Gregoire, F. M., Smas, C. M. & Sul, H. S. Understanding adipocyte differentiation. Physiol. Rev. 78, 783–809 (1998)
Djouadi, F. et al. A potential link between peroxisome proliferator-activated receptor signalling and the pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc. Res. 84, 83–90 (2009)
Son, N.-H. et al. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J. Clin. Invest. 117, 2791–2801 (2007)
Willson, T. M., Lambert, M. H. & Kliewer, S. A. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu. Rev. Biochem. 70, 341–367 (2001)
Waku, N. et al. The nuclear receptor PPARγ individually responds to serotonin- and fatty acid-metabolites. EMBO J. 29, 3395–3407 (2010)
Scolletta, S. & Biagioli, B. Energetic myocardial metabolism and oxidative stress: let's make them our friends in the fight against heart failure. Biomed. Pharmacother. 64, 203–207 (2010)
Ferrick, D. A., Neilson, A. & Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 13, 268–274 (2008)
Neubauer, S. The failing heart — an engine out of fuel. N. Engl. J. Med. 356, 1140–1151 (2007)
Kléber, A. G. & Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 84, 431–488 (2004)
Qyang, Y. et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell 1, 165–179 (2007)
Cai, C. L. et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5, 877–889 (2003)
Lombardi, R. et al. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ. Res. 104, 1076–1084 (2009)
Kattman, S. J. et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240 (2011)
Kita-Matsuo, H. et al. Lentiviral vectors and protocols for creation of stable hesc lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS ONE 4, e5046 (2009)
Nagy, L. et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 93, 229–240 (1998)
Dhalla, N. S., Elmoselhi, A. B., Hata, T. & Makino, N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 47, 446–456 (2000)
Grynkiewicz, G., Poenie, M. & Tsien, R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985)
Seahorse BioScience. http://www.seahorsebio.com/learning/tech-writing.php
Acknowledgements
We thank the patients for their participation, microarray core facilities at SBMRI and Scripps Research Institute for their support, T. Yi for technical assistance, and G. W. Rogers for assistance in metabolic assays. This work was supported by NIH grants (RO1 HL058493 and RO1 HL101189) (to D.P.K.); NIH grants (RO1 AR056712 and RO1 AR052779) (to P.L.P); California Institute of Regenerative Medicine (CIRM) grants (RS1-00171-1, RB2-01512 and RB4-06276) and NIH grant (RO1 HL105194) (to H.-S.V.C.).
Author information
Authors and Affiliations
Contributions
C.K. and H.-S.V.C. designed experiments and wrote the manuscript; J.E.M., H.C. and D.P.J. provided clinical assessment and patient’s fibroblasts as well as analysis of data and assistance with preparation of the manuscript; C.K., J.We., S.W., C.W., S.S., N.G.K. and H.-S.V.C. performed the experiments; S.F. and P.L.P. helped C.K. in performing bisulphide sequencing; J.Wo. analysed microarray, Seahorse and immunocytochemical data; D.P.K. and T.C.L. provided scientific advice and primers for metabolic assays; C.K., J.Wo. and H.-S.V.C performed and interpreted the calcium imaging data; all authors read and approved the manuscript, and H.-S.V.C. supervised the entire research project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This fie contains a more detailed legend for Figure 4, Supplementary Figures 1-16, Supplementary Tables 1-4, Supplementary Methods and Supplementary References. (PDF 21705 kb)
Supplementary Data
This file contains the microarray data used to construct figure 1f. (XLS 6324 kb)
A beating 70 day-old H9 hESC-EB
A beating embryoid body derived from H9 human embryonic stem cells after it has been cultured for 70 days in cardiogenic media. (AVI 3116 kb)
A beating 70 day-old c.2484C>T mutant PKP2 iPSC-EB
A beating embryoid body derived from the homozygous c.2484C>T mutant PKP2-induced pluripotent stem cells of patient #1 after it has been cultured for 70 days in cardiogenic media. (AVI 2958 kb)
Rights and permissions
About this article
Cite this article
Kim, C., Wong, J., Wen, J. et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 494, 105–110 (2013). https://doi.org/10.1038/nature11799
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11799
This article is cited by
-
Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy
Nature Cardiovascular Research (2023)
-
Arrhythmogenic Cardiomyopathy: from Preclinical Models to Genotype–phenotype Correlation and Pathophysiology
Stem Cell Reviews and Reports (2023)
-
Genetic lineage tracing identifies cardiac mesenchymal-to-adipose transition in an arrhythmogenic cardiomyopathy model
Science China Life Sciences (2023)
-
Synergistic effects of hormones on structural and functional maturation of cardiomyocytes and implications for heart regeneration
Cellular and Molecular Life Sciences (2023)
-
Impaired Plakophilin-2 in obesity breaks cell cycle dynamics to breed adipocyte senescence
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.