Abstract
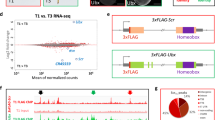
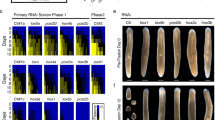
Homeotic (Hox) genes code for principal transcriptional regulators of animal body regionalization1. The duplication and divergence of Hox genes, changes in their regulation, and changes in the regulation of Hox target genes have all been implicated in the evolution of animal diversity2,3,4. It is not known whether Hox proteins have also acquired new activities during the evolution of specific lineages. Amino-acid sequences outside the DNA-binding homeodomains of Hox orthologues diverge significantly. These sequence differences may be neutral with respect to protein function, or they could be involved in the functional divergence of Hox proteins and the evolutionary diversification of animals. Here, we identify a transcriptional repression domain in the carboxy-terminal region of the Drosophila Ultrabithorax (Ubx) protein. This domain is highly conserved among Ubx orthologues in other insects, but is absent from Ubx in other arthropods and onychophorans. The evolution of this domain may have facilitated the greater morphological diversification of posterior thoracic and anterior abdominal segments characteristic of modern insects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McGinnis, W. & Krumlauf, R. Homeobox genes and axial patterning. Cell 68, 283–302 (1992)
Carroll, S. Homeotic genes and the evolution of arthropods and chordates. Nature 376, 479–485 (1995)
Gellon, G. & McGinnis, W. Shaping animal body plans in development and evolution by modulation of Hox expression patterns. BioEssays 20, 116–125 (1998)
Carroll, S. B., Grenier, J. K. & Weatherbee, S. D. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design (Blackwell Science, Malden, Massachusetts, 2001)
McGinnis, N., Kuziora, M. A. & McGinnis, W. Human Hox-4.2 and Drosophila encode similar regulatory specificities in Drosophila embryos and larvae. Cell 63, 969–976 (1990)
Zhao, J. J., Lazzarini, R. A. & Pick, L. The mouse Hox-1.3 gene is functionally equivalent to the Drosophila Sex combs reduced gene. Genes Dev. 7, 343–354 (1993)
Bachiller, D., Macias, A., Dubuoule, D. & Morata, G. Conservation of a functional hierarchy between mammalian and insect Hox/HOM genes. EMBO J. 13, 1930–1941 (1994)
Zakany, J., Gerard, M., Favier, B., Potter, S. S. & Duboule, D. Functional equivalence and rescue among group 11 Hox gene products in vertebral patterning. Dev. Biol. 176, 325–328 (1996)
Greer, J. M., Puetz, J., Thomas, K. R. & Capecchi, M. R. Maintenance of functional equivalence during paralogous Hox gene evolution. Nature 403, 661–665 (2000)
Grenier, J. K. & Carroll, S. B. Functional evolution of the Ultrabithorax protein. Proc. Natl Acad. Sci. USA 97, 704–709 (2000)
Vachon, G. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene. Cell 71, 437–450 (1992)
Ronshaugen, M., McGinnis, N. & McGinnis, W. Hox protein mutation and macroevolution of the insect body plan. Nature advance online publication, 6 February 2002 (DOI 10.1038/nature716).
van Dijk, M. & Murre, C. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell 78, 617–624 (1994)
Chang, C.-P., Shen, W.-F., Rozenfeld, S. & Lawrence, H. J. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 9, 663–674 (1995)
Johnson, F. B., Parker, E. & Krasnow, M. A. Extradenticle protein is a selective cofactor for the Drosophila homeotics: role of the homeodomain and YPWM amino acid motif in the interaction. Proc. Natl Acad. Sci. USA 92, 739–743 (1995)
Manak, J. R., Mathies, L. D. & Scott, M. P. Regulation of a decapentaplegic midgut enhancer by homeotic proteins. Development 120, 3605–3619 (1994)
Rauskolb, C. & Wieschaus, E. Coordinate regulation of downstream genes by extradenticle and the homeotic selector proteins. EMBO J. 13, 3561–3569 (1994)
White, R. A. H., Aspland, S. E., Brookman, J. J., Clayton, L. & Sproat, G. The design and analysis of a homeotic response element. Mech. Dev. 91, 217–226 (2000)
Hanna-Rose, W. & Hansen, U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 12, 229–234 (1996)
Yeung, K., Kim, S. & Reinberg, D. Functional dissection of a human Dr1–DRAP1 repressor complex. Mol. Cell. Biol. 17, 36–45 (1997)
Janody, F., Sturny, R., Schaeffer, V., Azou, Y. & Dostatni, N. Two distinct domains of Bicoid mediate its transcriptional downregulation by the Torso pathway. Development 128, 2281–2290 (2001)
Gibson, G. Evolution: Hox genes and the cellared wine principle. Curr. Biol. 10, 452–455 (2000)
Alonso, C. R., Maxton-Kuechenmeister, J. & Akam, M. Evolution of Ftz protein function in insects. Curr. Biol. 11, 1473 (2001)
Löhr, U., Miyuki, Y. & Pick, L. Drosophila fushi tarazu: a gene on the border of homeotic function. Curr. Biol. 11, 1403 (2001)
Dearden, P. & Akam, M. Developmental evolution: Axial patterning in insects. Curr. Biol. 9, 591–594 (1999)
Bennett, R. L., Brown, S. J. & Denell, R. E. Molecular and genetic analysis of the Tribolium Ultrabithorax ortholog, Ultrathorax. Dev. Genes Evol. 209, 608–619 (1999)
Castelli-Gair, J., Greig, S., Micklem, G. & Akam, M. Dissecting the temporal requirements for homeotic gene function. Development 120, 1983–1985 (1994)
Brand, A. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993)
Kelsh, R., Weinzierl, R., White, R. & Akam, M. Homeotic gene expression in the locust Schistocerca: An antibody that detects conserved epitopes in Ultrabithorax and abdominal-A genes. Dev. Genet. 15, 19–31 (1994)
Dalton, S. & Treisman, R. Characterization of SAP-1, a protein recruited by serum response factor to the C-fos serum response element. Cell 68, 597 (1992)
Acknowledgements
We thank M. Ronshaugen and W. McGinnis for communication of results before publication. We thank D. Lewis for cloning and sequencing JcUbx; M. DeCamillis for providing Tribolium castaneum RNA; R. Mann for providing the Exd expression clone; N. Dostatni and A. Laughon for plasmids; J. Grenier for advice; K. Vorwerk and V. Kassner for technical support; A. Kopp, N. King and J. Grenier for comments; and J. Carroll for help with manuscript preparation. R.G. was supported by a National Institutes of Health predoctoral training grant provided to the Department of Genetics, and S.B.C. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Galant, R., Carroll, S. Evolution of a transcriptional repression domain in an insect Hox protein. Nature 415, 910–913 (2002). https://doi.org/10.1038/nature717
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature717
This article is cited by
-
Characterizing Hox genes in mayflies (Ephemeroptera), with Hexagenia limbata as a new mayfly model
EvoDevo (2022)
-
Molecular and evolutionary processes generating variation in gene expression
Nature Reviews Genetics (2021)
-
Amino acid homorepeats in proteins
Nature Reviews Chemistry (2020)
-
Constraints and consequences of the emergence of amino acid repeats in eukaryotic proteins
Nature Structural & Molecular Biology (2017)
-
Non-specificity of transcription factor function in Drosophila melanogaster
Development Genes and Evolution (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.