Abstract
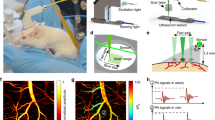
Imaging techniques based on optical contrast analysis can be used to visualize dynamic and functional properties of the nervous system via optical signals resulting from changes in blood volume, oxygen consumption and cellular swelling associated with brain physiology and pathology. Here we report in vivo noninvasive transdermal and transcranial imaging of the structure and function of rat brains by means of laser-induced photoacoustic tomography (PAT). The advantage of PAT over pure optical imaging is that it retains intrinsic optical contrast characteristics while taking advantage of the diffraction-limited high spatial resolution of ultrasound. We accurately mapped rat brain structures, with and without lesions, and functional cerebral hemodynamic changes in cortical blood vessels around the whisker-barrel cortex in response to whisker stimulation. We also imaged hyperoxia- and hypoxia-induced cerebral hemodynamic changes. This neuroimaging modality holds promise for applications in neurophysiology, neuropathology and neurotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Grinvald, A., Frostig, R.D., Lieke, E. & Hildesheim, R. Optical imaging of neuronal activity. Physiol. Rev. 68, 1285–1365 (1988).
Frostig, R.D., Lieke, E.E., Ts'o, D.Y. & Grinvald, A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high resolution optical imaging of intrinsic signals. Proc. Natl. Acad. Sci. USA 87, 6082–6086 (1990).
MacVicar, B.A. & Hochman, D. Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J. Neurosci. 11, 1458–1469 (1991).
Ebner, T.J. & Chen, G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog. Neurobiol. 46, 463–506 (1995).
Villringer, A. & Chance, B. Non-invasive optical spectroscopy and imaging of human brain function. Trends. Neurosci. 20, 435–442 (1997).
Mayhew, J. et al. Spectroscopic analysis of changes in remitted illumination: the response to increased neural activity in brain. Neuroimage 10, 304–326 (1999).
Nemoto, M. et al. Analysis of optical signals evoked by peripheral nerve stimulation in rat somatosensory cortex: dynamic changes in hemoglobin concentration and oxygenation. J. Cereb. Blood Flow Metab. 19, 246–259 (1999).
Gratton, G. & Fabiani, M. Dynamic brain imaging: event-related optical signals (EROS) measures of the time course and localization of cognitive-related activity. Psychon. Bull Rev. 5, 535–563 (1998).
Grinvald, A., Lieke, E., Frostig, R.D., Gilbert, C.D. & Wiesel, T.N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324, 361–364 (1986).
Haglund, M.M., Ojemann, G.A. & Hochman, D.W. Optical imaging of epileptiform and functional activity in human cerebral cortex. Nature 358, 668–671 (1992).
Dowling, J.L., Henegar, M.M., Liu, D., Rovainen, C.M. & Woolsey, T.A. Rapid optical imaging of whisker responses in the rat barrel cortex. J. Neurosci. Meth. 66, 113–122 (1996).
Jones, M., Berwick, J. & Mayhew, J. Changes in blood flow, oxygenation, and volume following extended stimulation of rodent barrel cortex. Neuroimage 15, 474–487 (2002).
Hoelen, C.G.A., de Mul, F.F.M., Pongers, R. & Dekker, A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Opt. Lett. 23, 648–650 (1998).
Kruger, R.A., Reinecke, D.R. & Kruger, G.A. Thermoacoustic computed tomography–technical considerations. Med. Phys. 26, 1832–1837 (1999).
Esenaliev, R.O., Karabutov, A.A. & Oraevsky, A.A. Sensitivity of laser opto-acoustic imaging in detection of small deeply embedded tumors. IEEE J. Sel. Top. Quant. 5, 981–988 (1999).
Paltauf, G. & Schmidt-Kloiber, H. Optical method for two-dimensional ultrasonic detection. Appl. Phys. Lett. 75, 1048–1050 (1999).
Karabutov, A.A., Savateeva, E. & Podymova, N.B. Backward mode detection of laser-induced wide-band ultrasonic transients with optoacoustic transducer. J. Appl. Phys. 87, 2003–2014 (2000).
Köstli, K.P. et al. Optoacoustic imaging using a three-dimensional reconstruction algorithm. IEEE J. Sel. Top. Quant. 7, 918–923 (2001).
Wang, X. et al. Photoacoustic tomography of biological tissues with high cross-section resolution: reconstruction and experiment. Med. Phys. 29, 2799–2805 (2002).
Tokuno, H., Hatanaka, N., Takada, M. & Nambu, A. B-mode and color Doppler ultrasound imaging for localization of microelectrode in monkey brain. Neurosci. Res. 36, 335–338 (2000).
Diebold, G.J., Sun, T. & Khan, M.I. in Photoacoustic and Photothermal Phenomena III (ed. Bicanic, D.) 263–296 (Springer, Berlin, Heidelberg, 1992).
Sun, T. & Diebold, G.J. Generation of ultrasonic waves from a layered photoacoustic source. Nature 355, 806–808 (1992).
Xu, M. & Wang, L.V. Time-domain reconstruction for thermoacoustic tomography in a spherical geometry. IEEE T. Med. Imaging 21, 814–822 (2002).
Gerrits, R.J., Stein, E.A. & Greene, A.S. Blood flow increases linearly in rat somatosensory cortex with increased whisker movement frequency. Brain Res. 783, 151–157 (1998).
Ogawa, S. et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Nat. Acad. Sci. USA 89, 5951–5955 (1992).
Hoge, R.D. et al. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 42, 849–863 (1999).
Kety, S.S. & Schmidt, C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 27, 484–491 (1948).
Siesjo, B. Brain Energy Metabolism (John Wiley, New York, 1978).
Bereczki, D. et al. Hypoxia increases velocity of blood-flow through parenchymal microvascular systems in rat brain. Cerebr. Blood F. Met. 13, 475–486 (1993).
Duong, T.Q., Iadecola, C. & Kim, S.G. Effect of hyperoxia, hypercapnia, and hypoxia on cerebral interstitial oxygen tension and cerebral blood flow. Magn. Reson. Med. 45, 61–70 (2001).
Millikan, G.A. The oximeter, an instrument for measuring continuously the oxygen saturation of arterial blood in man. Rev. Sci. Instrum. 13, 434–444 (1942).
Jöbsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 (1977).
Mayevsky, A. & Chance, B. Intracellular oxidation-reduction state measured in situ by a multichannel fiberoptic surface fluorometer. Science 217, 537–540 (1982).
Vanderlo, H. & Woolsey, T.A. Somatosensory cortex-structural alterations following early injury to sense organs. Science 179, 395–398 (1973).
US National Institutes of Health. Guide for the Care and Use of Laboratory Animals, NIH Publication No. 86-23 (US Government Printing Office, Washington, DC, USA, 1985).
Acknowledgements
This research was supported in part by the US Department of Defense, National Institutes of Health, National Science Foundation, and Texas Advanced Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, X., Pang, Y., Ku, G. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat Biotechnol 21, 803–806 (2003). https://doi.org/10.1038/nbt839
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt839
This article is cited by
-
Parallel interrogation of the chalcogenide-based micro-ring sensor array for photoacoustic tomography
Nature Communications (2023)
-
Spiral volumetric optoacoustic tomography for imaging whole-body biodynamics in small animals
Nature Protocols (2023)
-
Label-free biomedical optical imaging
Nature Photonics (2023)
-
A sound solution for deep-brain imaging
Nature Methods (2023)
-
Photoacoustic Microscopic Imaging of Cerebral Vessels for Intensive Monitoring of Metabolic Acidosis
Molecular Imaging and Biology (2023)