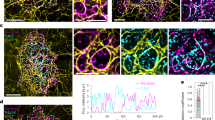
Abstract
Sphingosylphosphorylcholine (SPC) is a naturally occurring bioactive lipid that is present in high density lipoproteins (HDL) particles and found at increased levels in blood and malignant ascites of patients with ovarian cancer. Here, we show that incubation of human epithelial tumour cells with SPC induces a perinuclear reorganization of intact keratin 8–18 filaments. This effect is specific for SPC, largely independent of F-actin and microtubules, and is accompanied by keratin phosphorylation. In vivo visco-elastic probing of single cancer cells demonstrates that SPC increases cellular elasticity. Accordingly, SPC stimulates migration of cells through size-limited pores in a more potent manner than lysophosphatidic acid (LPA). LPA induces actin stress fibre formation, but does not reorganize keratins in cancer cells and hence increases cellular stiffness. We propose that reorganization of keratin by SPC may facilitate biological phenomena that require a high degree of elasticity, such as squeezing of cells through membranous pores during metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Seufferlein, T. & Rozengurt, E. Sphingosylphosphorylcholine activation of mitogen-activated protein kinase in Swiss 3T3 cells requires protein kinase C and pertussis toxin-sensitive G protein. J. Biol. Chem. 270, 24334–24342 (1995).
Boguslawski, G., Lyons, D., Harvey, K.A., Kovala, A.T. & English, D. Sphingosylphosphorylcholine induces endothelial cell migration and morphogenesis. Biochem. Biophys Res. Commun 272, 603–609 (2000).
Wakita H et al. Sphingosylphosphorylcholine stimulates proliferation and upregulates cell surface-associated plasminogen activator activity in cultured human keratinocytes. J. Invest. Dermatol. 110, 253–258 (1998).
An S et al. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 417, 279–282 (1997).
Xu, Y. et al. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nature Cell Biol. 2, 261–267 (2000).
Zhu, K. et al. Sphingosylphosphorylcholine (SPC) and lysophosphatidylcholine (LPC) are ligands for GPR4. J. Biol. Chem. 276, 41325–41335 (2001).
Rodriguez-Lafrasse, C. & Vanier, M.T. Sphingosylphosphorylcholine in Niemann-Pick disease brain: accumulation in type A but not in type B. Neurochem. Res. 24, 199–205 (1999).
Liliom, K. et al. Sphingosylphosphocholine is a naturally occuring lipid mediator in blood plasma: a possible role in regulating cardiac function via sphingolipid receptors. Biochem. J. 355, 189–197 (2001).
Nofer, J.-R. et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J. Biol. Chem. 276, 34480–34485 (2001).
Xiao, Y.J. et al. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem. 290, 302–313 (2001).
Ballestrem, C., Wehrle-Haller, B., Hinz, B. & Imhof, B.A. Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control directed cell migration. Mol. Biol Cell. 11, 2999–3012 (2000).
Fuchs, E. & Weber, K. Intermediate filaments: structure, dynamics, function and disease. Annu. Rev. Biochem. 63, 345–382 (1994).
Omary, M.B., Ku, N.-O., Liao, J. & Price, D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcellular Biochem. 31, 105–140 (1998).
Hatzfeld, M. & Franke, W.W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J. Cell. Biol. 101, 1826–1841 (1985).
Yamada, S., Wirtz, D. & Coulombe, P.A. Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol. Biol. Cell. 13, 382–391 (2002).
Oshima, R.G., Baribault, H. & Caulin, C. Oncogenic regulation and function of keratin 8 and 18. Cancer Metastasis Rev. 15, 445–471 (1996).
Fuchs, E. & Cleveland, D.W. A structural scaffolding of intermediate filaments in health and disease. Science 279, 514–519 (1998).
Maniotis, A.J., Chen, C.S. & Ingber, D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. USA 94, 849–854 (1997).
Santini, D. et al. Expression of intermediate filaments in normal and neoplastic exocrine pancreas. Gastroenterology 106, 1326–1332 (1994).
Caulin, C., Salvesen, G.S. & Oshima, R.G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell. Biol. 138, 1379–1394 (1997).
Toivola, D.M., Goldman, R.D., Garrod, D.R. & Eriksson, J.E. Protein phosphatases maintain the organization and structural interaction of hepatic keratin intermediate filaments. J. Cell Sci. 110, 23–33 (1997).
Yoon, M., Moir, R.D., Prahlad, V. & Goldman, R.D. Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 (1998).
Windoffer, R. & Leube, R.E. Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J. Cell. Sci. 112, 4521–4534 (1999).
Hofmann, I. & Franke, W.W. Heterotypic interactions and filament assembly of type I and type II cytokeratins in vitro: viscometry and determinations of relative affinities. Eur. J. Cell Biol. 72, 122–132 (1997).
Hesse, M., Franz, T., Tamai, Y., Taketo, M.M. & Magin, T.M. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 19, 5060–5070 (2000).
Merkel, R., Nassoy, P., Leung, A., Ritchie, K. & Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397, 50–53 (1999).
Rumenapp, U. et al. Sphingolipid receptor signaling and function in human bladder carcinoma cells: inhibition of LPA- but enhancement of thrombin-stimulated cell motility. Naunyn Schmiedebergs Arch Pharmacol 361, 1–11 (2000).
Ma, L., Yamada, S., Wirtz, D. & Coulombe, P.A. A hot-spot mutation alters the mechanical properties of keratin filament networks. Nature Cell Biol 3, 503–506 (2001).
Goldman, R.D., Khuon, S., Chou, Y.H., Opal, P. & Steinert, P.M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J. Cell Biol. 134, 971–983 (1996).
Chu, Y.W., Runyan, R.B., Oshima, R.G. & Hendrix, M.J.C. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc. Natl Acad. Sci. USA 90, 4261–4265 (1993).
Paladini, R.D., Takahashi, K., Bravo, N.S. & Coulombe, P.A. Onset of Re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J. Cell Biol. 132, 381–397 (1996).
Svitkina, T.M. & Borisi, G.G. Correlative light and electron microscopy of the cytoskeleton of cultured cells. Methods Enzymol. 298, 570–592 (1998).
Thoumine, O. & Ott, A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J. Cell Sci. 110, 2109–2116 (1997).
Van Veldhoven, P.P., Foglesong, R.J. & Bell, R.M. A facile enzymatic synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. J. Lipid Res. 30, 611–616 (1989).
De Ceuster, P., Mannaerts, G.P. & Van Veldhoven, P.P. Identification and subcellular localization of sphinganine-phosphatases in rat liver. Biochem J. 311, 139–146 (1995).
Acknowledgements
U. Nolte is acknowledged for expert technical assistance. M.B., T.S., A.M. and J.S. are supported by the Deutsche Forschungsgemeinschaft (SFB 518/ A5 to M.B. and B3 to T.S., SP520/5 to A.M. and J.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Fig. S1
High-resolution SEM of keratin filaments in Panc-1 cells A: Unstimulated Panc-1 cells underwent a prefixation treatment to extract individual keratin filaments for visualisation by SEM. These filaments were identified as keratin 8 and 18 by immunogold labelling. 5 nm gold particles identifying keratin 8/18 specific antibodies are presented in black. The scale bar shown represents 200 nm. B: The density of keratin filaments in the perinuclear area in response to 10 μM SPC or solvent was quantified by counting the number of filaments crossing a 5 μm line. In each condition, this line was placed randomly around ten different nuclei. Shown are the means +/- S.D. of three independent experiments. (PDF 398 kb)
Supplementary Information, Fig. S2
SPC-induced keratin reorganisation is independent of other cytoskeletal components A: Immunofluorescence of VASP in Panc-1 cells after treatment with 10 μM SPC or 10 μM LPA for 45 min or solvent (-). B: Panc-1 cells were treated with 1.5 μM cytochalasin D (CytD, +), 50 nM jasplakinolide (Jas, +) for 2h. Cells were subsequently stimulated with 10 µM SPC for 45 min (SPC, +). Keratin immunostaining was performed using the KL1 antibody, F-actin was visualised by Oregon-green-phallodin. C: Panc-1 cells were incubated with 1 µM nocodazol (Noc) or 5 μM taxol (Tax) for 1h and subsequently stimulated with 10 μM SPC for 45 min (SPC, +). Tubulin and keratin immunostaining was performed with anti-a-tubulin and KL1 antibodies, respectively. The scale bars shown represent 5 μm. (PDF 3228 kb)
Supplementary Information, Fig. S3
Experimental set-up for the force measurements (PDF 42 kb)
Rights and permissions
About this article
Cite this article
Beil, M., Micoulet, A., von Wichert, G. et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol 5, 803–811 (2003). https://doi.org/10.1038/ncb1037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1037
This article is cited by
-
The laminin–keratin link shields the nucleus from mechanical deformation and signalling
Nature Materials (2023)
-
A reliable elasticity sensing method for analysis of cell entosis using microfluidic cytometer
Biomedical Engineering Letters (2023)
-
A hierarchical cellular structural model to unravel the universal power-law rheological behavior of living cells
Nature Communications (2021)
-
Novel K6-K14 keratin fusion enhances cancer stemness and aggressiveness in oral squamous cell carcinoma
Oncogene (2019)
-
GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol
Acta Pharmacologica Sinica (2019)