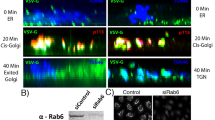
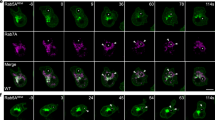
Abstract
Integrating the pleomorphic membranes of the intermediate compartment (IC) into the array of Golgi cisternae is a crucial step in membrane transport, but it is poorly understood. To gain insight into this step, we investigated the dynamics by which cis-Golgi matrix proteins such as GM130 and GRASP65 associate with, and incorporate, incoming IC elements. We found that GM130 and GRASP65 cycle via membranous tubules between the Golgi complex and a constellation of mobile structures that we call late IC stations. These stations are intermediate between the IC and the cis-Golgi in terms of composition, and they receive cargo from earlier IC elements and deliver it to the Golgi complex. Late IC elements are transient in nature and sensitive to fixatives; they are seen in only a fraction of fixed cells, whereas they are always visible in living cells. Finally, late IC stations undergo homotypic fusion and establish tubular connections between themselves and the Golgi. Overall, these features indicate that late IC stations mediate the transition between IC elements and the cis-Golgi face.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Godi, A. et al. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc. Natl Acad. Sci. USA 95, 8607–8612 (1998).
Godi, A. et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nature Cell Biol. 1, 280–287 (1999).
De Matteis, M. A. & Morrow, J. S. Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 113, 2331–2343 (2000).
Nakamura, N. et al. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131, 1715–1726 (1995).
Slusarewicz, P., Nilsson, T., Hui, N., Watson, R. & Warren, G. Isolation of a matrix that binds medial Golgi enzymes. J. Cell Biol. 124, 405–413 (1994).
Barr, F. A., Puype, M., Vandekerckhove, J. & Warren, G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91, 253–262 (1997).
Shorter, J. & Warren, G. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J. Cell Biol. 146, 57–70 (1999).
Sonnichsen, B. et al. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140, 1013–1021 (1998).
Seemann, J., Jokitalo, E., Pypaert, M. & Warren, G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature 407, 1022–1026 (2000).
Klumperman, J. Transport between ER and Golgi. Curr. Opin. Cell Biol. 12, 445–449 (2000).
Krijnse-Locker, J., Ericsson, M., Rottier, P. J. & Griffiths, G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 124, 55–70 (1994).
Sitia, R. & Meldolesi, J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol. Biol. Cell 3, 1067–1072 (1992).
Mellman, I. & Simons, K. The Golgi complex: in vitro veritas? Cell 68, 829–840 (1992).
Bannykh, S. I. & Balch, W. E. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J. Cell Biol. 138, 1–4 (1997).
Lotti, L. V., Torrisi, M. R., Pascale, M. C. & Bonatti, S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J. Cell Biol. 118, 43–50 (1992).
Pelham, H. R. Control of protein exit from the endoplasmic reticulum. Annu. Rev. Cell Biol. 5, 1–23 (1989).
Saraste, J. & Svensson, K. Distribution of the intermediate elements operating in ER to Golgi transport. J. Cell Sci. 100, 415–430 (1991).
Ying, M., Flatmark, T. & Saraste, J. The p58-positive pre-golgi intermediates consist of distinct subpopulations of particles that show differential binding of COPI and COPII coats and contain vacuolar H(+)-ATPase. J. Cell Sci. 113, 3623–3638 (2000).
Presley, J. F. et al. ER-to-Golgi transport visualized in living cells. Nature 389, 81–85 (1997).
Scales, S. J., Pepperkok, R. & Kreis, T. E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90, 1137–1148 (1997).
Hauri, H. P., Kappeler, F., Andersson, H. & Appenzeller, C. ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 113, 587–596 (2000).
Hammond, A. T. & Glick, B. S. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol. Biol. Cell 11, 3013–3030 (2000).
Shima, D. T., Scales, S. J., Kreis, T. E. & Pepperkok, R. Segregation of COPI-rich and anterograde-cargo-rich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr. Biol. 9, 821–824 (1999).
Stephens, D. J., Lin-Marq, N., Pagano, A., Pepperkok, R. & Paccaud, J. P. COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J. Cell Sci. 113, 2177–2185 (2000).
Perez-Vilar, J., Hidalgo, J. & Velasco, A. Presence of terminal N-acetylgalactosamine residues in subregions of the endoplasmic reticulum is influenced by cell differentiation in culture. J. Biol. Chem. 266, 23967–23976 (1991).
Klumperman, J. et al. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 111, 3411–3425 (1998).
Tang, B. L., Low, S. H., Hauri, H. P. & Hong, W. Segregation of ERGIC53 and the mammalian KDEL receptor upon exit from the 15 degrees C compartment. Eur. J. Cell Biol. 68, 398–410 (1995).
Mironov, A. A., Polishchuk, R. S. & Luini, A. Visualizing membrane traffic in vivo by combined video fluorescence and 3D electron microscopy. Trends Cell Biol. 10, 349–353 (2000).
Powell, R. D., Halsey, C. M. & Hainfeld, J. F. Combined fluorescent and gold immunoprobes: reagents and methods for correlative light and electron microscopy. Microsc. Res. Technol. 42, 2–12 (1998).
Kuismanen, E. & Saraste, J. Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol. 32, 257–274 (1989).
Barroso, M., Nelson, D. S. & Sztul, E. Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc. Natl Acad. Sci. USA 92, 527–531 (1995).
Waters, M. G., Clary, D. O. & Rothman, J. E. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol. 118, 1015–1026 (1992).
Alvarez, C., Garcia-Mata, R., Hauri, H. P. & Sztul, E. The p-115 inteactive proteins, GM130 and giantin participate in ER-Golgi traffic. J. Biol. Chem. 276, 2693–2700 (2001).
Seemann, J., Jokitalo, E. J. & Warren, G. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell 11, 635–645 (2000).
Barr, F. A., Nakamura, N. & Warren, G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 17, 3258–3268 (1998).
Nakamura, N., Lowe, M., Levine, T. P., Rabouille, C. & Warren, G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89, 445–455 (1997).
Alvarez, C., Fujita, H., Hubbard, A. & Sztul, E. ER to Golgi transport: Requirement for p115 at a pre-Golgi VTC stage. J. Cell Biol. 147, 1205–1222 (1999).
Garcia-Mata, R., Gao, Y., Alvarez, C. & Sztul, E. S. The membrane transport factor p115 recycles only between homologous compartments in intact heterokaryons. Eur. J. Cell Biol. 79, 229–239 (2000).
Hopkins, C. R., Gibson, A., Shipman, M. & Miller, K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature 346, 335–339 (1990).
Blum, R., Stephens, D. J. & Schulz, I. Lumenal targeted GFP, used as a marker of soluble cargo, visualises rapid ERGIC to golgi traffic by a tubulo-vesicular network. J. Cell Sci. 113, 3151–3159 (2000).
Moyer, B. D., Allan, B. B. & Balch, W. E. Rab1 Interaction with a GM130 Effector Complex Regulates COPII Vesicle cis-Golgi Tethering. Traffic 2, 268–276 (2001).
Chao, D. S. et al. SNARE membrane trafficking dynamics in vivo. J. Cell Biol. 144, 869–881 (1999).
Hay, J. C. et al. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol. 141, 1489–1502 (1998).
Weide, T. et al. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2, 336–341 (2001).
Allan, B. B., Moyer, B. D. & Balch, W. E. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448 (2000).
Fritzler, M. J., Hamel, J. C., Ochs, R. L. & Chan, E. K. Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J. Exp. Med. 178, 49–62 (1993).
Gallione, C. J. & Rose, J. K. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J. Virol. 54, 374–382 (1985).
Mironov, A. et al. Role of NAD+ and ADP-ribosylation in the maintenance of the Golgi structure. J. Cell Biol. 139, 1109–1118 (1997).
Peters, P. J. & Hunziker, W. Subcellular localization of Rab17 by cryo-immunogold electron microscopy in epithelial cells grown on polycarbonate filters. Methods Enzymol. 329, 210–225 (2001).
Acknowledgements
We thank P. Hauri, J. Gruenberg, B. L. Tang, J. Lippincott-Schwartz and F. Barr for antibodies, C. P. Berrie for comments on the manuscript, and Y. Misumi for G95 constructs. Supported in part by grants from Telethon (E.732), the Human Frontier Science Programme (to M.A.D.M.), the European Community (EU grant No. HPRN-CT-2000–00081), the Italian National Research Council (99.00127.PF49 and 99.00133.PF49), and the Italian Association for Cancer Research (AIRC, Milan, Italy). The human GM130 nucleotide sequence data has been submitted to the EMBL/GenBank/DDBJ data libraries with accession number D86427.
Author information
Authors and Affiliations
Supplementary information
Movie 1
Dynamics of GFP-GM130. Time laps images from COS7 cells transfected with GFP-GM130. The numbers in the bottom right corner designate frame numbers and time elapsed in h.mm.ss., respectively. (MOV 6310 kb)
Movie 2
Dynamics of GFP-GM130. Time laps images from COS7 cells transfected with GFP-GM130. The numbers in the bottom left corner designate frame numbers and time elapsed in h.mm.ss., respectively. (MOV 5616 kb)
Movie 3
Dynamics of YFP-GM130 and CFP-VSV-G upon release from the 15 °C block. Time laps images images from COS7 cells transfected with YFP-GM130 (red) and CFP-VSV-G (green) showing the fluorescence recovery after photobleaching of the central Golgi area (white circle) upon release from the 15 °C block. (MOV 6460 kb)
Movie 4
Dynamics of GFP-G95. Time laps images from COS7 cells transfected with GFP-G95. The numbers in the bottom left corner designate frame numbers and time elapsed in h.mm.ss., respectively. (MOV 1082 kb)
Rights and permissions
About this article
Cite this article
Marra, P., Maffucci, T., Daniele, T. et al. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat Cell Biol 3, 1101–1113 (2001). https://doi.org/10.1038/ncb1201-1101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1201-1101
This article is cited by
-
Cdk1 protects against oxygen-glucose deprivation and reperfusion-induced Golgi fragmentation and apoptosis through mediating GM130 phosphorylation
Journal of Molecular Histology (2023)
-
The role of NSP6 in the biogenesis of the SARS-CoV-2 replication organelle
Nature (2022)
-
Cellular and subcellular localization of endogenous phospholipase D6 in seminiferous tubules of mouse testes
Cell and Tissue Research (2021)
-
Distributed synthesis of sarcolemmal and sarcoplasmic reticulum membrane proteins in cardiac myocytes
Basic Research in Cardiology (2021)
-
Roles of N-glycans in the polymerization-dependent aggregation of mutant Ig-μ chains in the early secretory pathway
Scientific Reports (2017)