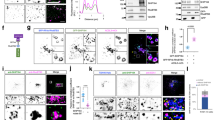
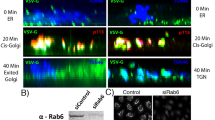
Abstract
Here we identify a new regulator of endocytosis called RME-6. RME-6 is evolutionarily conserved among metazoans and contains Ras-GAP (GTPase-activating protein)-like and Vps9 domains. Consistent with the known catalytic function of Vps9 domains in Rab5 GDP/GTP exchange, we found that RME-6 binds specifically to Caenorhabditis elegans RAB-5 in the GDP-bound conformation, and rme-6 mutants have phenotypes that indicate low RAB-5 activity. However, unlike other Rab5-associated proteins, a rescuing green fluorescent protein (GFP)–RME-6 fusion protein primarily localizes to clathrin-coated pits, physically interacts with α-adaptin, a clathrin adaptor protein, and requires clathrin to achieve its cortical localization. In rme-6 mutants, transport from the plasma membrane to endosomes is defective, and small 110-nm endocytic vesicles accumulate just below the plasma membrane. These results suggest a mechanism for the activation of Rab5 in clathrin-coated pits or clathrin-coated vesicles that is essential for the delivery of endocytic cargo to early endosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Brodsky, F.M., Chen, C.Y., Knuehl, C., Towler, M.C. & Wakeham, D.E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568 (2001).
Zerial, M. & McBride, H. Rab proteins as membrane organizers. Nature Rev. Mol. Cell Biol. 2, 107–117 (2001).
Chavrier, P., Parton, R.G., Hauri, H.P., Simons, K. & Zerial, M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317–329 (1990).
Bucci, C. et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715–728 (1992).
Christoforidis, S., McBride, H.M., Burgoyne, R.D. & Zerial, M. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621–625 (1999).
McBride, H.M. et al. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98, 377–386 (1999).
Nielsen, E., Severin, F., Backer, J.M., Hyman, A.A. & Zerial, M. Rab5 regulates motility of early endosomes on microtubules. Nature Cell Biol. 1, 376–382 (1999).
de Renzis, S., Sonnichsen, B. & Zerial, M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nature Cell Biol. 4, 124–133 (2002).
Horiuchi, H. et al. A novel rab5 GDP/GTP exchange factor complexed to rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159 (1997).
Rubino, M., Miaczynska, M., Lippe, R. & Zerial, M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J. Biol. Chem. 275, 3745–3748 (2000).
McLauchlan, H. et al. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 8, 34–45 (1998).
Delprato, A., Merithew, E. & Lambright, D.G. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell 118, 607–617 (2004).
Tall, G.G., Barbieri, M.A., Stahl, P.D. & Horazdovsky, B.F. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82 (2001).
Topp, J.D., Gray, N.W., Gerard, R.D. & Horazdovsky, B.F. Alsin is a Rab5 and Rac1 guanine nucleotide exchange factor. J. Biol. Chem. 279, 24612–24623 (2004).
Horiuchi, H., Giner, A., Hoflack, B. & Zerial, M. A GDP/GTP exchange-stimulatory activity for the Rab5-RabGDI complex on clathrin-coated vesicles from bovine brain. J. Biol. Chem. 270, 11257–11262 (1995).
Grant, B. & Hirsh, D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326 (1999).
Fares, H. & Greenwald, I. Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 159, 133–145 (2001).
Bernards, A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603, 47–82 (2003).
Hajnal, A., Whitfield, C.W. & Kim, S.K. Inhibition of Caenorhabditis elegans vulval induction by gap-1 and by let-23 receptor tyrosine kinase. Genes Dev. 11, 2715–2728 (1997).
Hayashizaki, S., Iino, Y. & Yamamoto, M. Characterization of the C. elegans gap-2 gene encoding a novel Ras-GTPase activating protein and its possible role in larval development. Genes Cells 3, 189–202 (1998).
Hart, M.J., Callow, M.G., Souza, B. & Polakis, P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 15, 2997–3005 (1996).
Hama, H., Tall, G.G. & Horazdovsky, B.F. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J. Biol. Chem. 274, 15284–15291 (1999).
Stenmark, H., Vitale, G., Ullrich, O. & Zerial, M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 83, 423–432 (1995).
Zhang, Y., Grant, B. & Hirsh, D. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol. Biol. Cell 12, 2011–2021 (2001).
Greener, T. et al. Caenorhabditis elegans auxilin: a J-domain protein essential for clathrin-mediated endocytosis in vivo. Nature Cell Biol. 3, 215–219 (2001).
Fares, H. & Grant, B. Deciphering endocytosis in Caenorhabditis elegans. Traffic 3, 11–19 (2002).
Pellettieri, J., Reinke, V., Kim, S.K. & Seydoux, G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev. Cell 5, 451–462 (2003).
Praitis, V., Casey, E., Collar, D. & Austin, J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217–1226 (2001).
Barbieri, M.A. et al. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J. Cell Biol. 151, 539–550 (2000).
Galperin, E. & Sorkin, A. Visualization of Rab5 activity in living cells by FRET microscopy and influence of plasma-membrane-targeted Rab5 on clathrin-dependent endocytosis. J. Cell Sci. 116, 4799–4810 (2003).
Christoforidis, S. et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biol. 1, 249–252 (1999).
Shiba, Y., Takatsu, H., Shin, H.W. & Nakayama, K. Gamma-adaptin interacts directly with Rabaptin-5 through its ear domain. J. Biochem. (Tokyo) 131, 327–336 (2002).
Mattera, R., Arighi, C.N., Lodge, R., Zerial, M. & Bonifacino, J.S. Divalent interaction of the GGAs with the Rabaptin-5–Rabex-5 complex. EMBO J. 22, 78–88 (2003).
Mishra, S.K. et al. Dual-engagement regulation of protein interactions with the AP-2 adaptor alpha appendage. J. Biol. Chem. (2004).
Han, M. & Sternberg, P.W. let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell 63, 921–931 (1990).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Dang, H., Li, Z., Skolnik, E.Y. & Fares, H. Disease-related myotubularins function in endocytic traffic in Caenorhabditis elegans. Mol. Biol. Cell 15, 189–196 (2004).
Kamath, R.S. & Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30, 313–321 (2003).
Timmons, L., Court, D.L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001).
Williams, B.D., Schrank, B., Huynh, C., Shownkeen, R. & Waterston, R.H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131, 609–624 (1992).
Mello, C.C., Kramer, J.M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Campbell, R.E. et al. A monomeric red fluorescent protein. Proc. Natl Acad. Sci. USA 99, 7877–7882 (2002).
Fares, H. & Greenwald, I. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nature Genet. 28, 64–68 (2001).
Grant, B. et al. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nature Cell Biol. 3, 573–579 (2001).
Shaye, D.D. & Greenwald, I. Endocytosis-mediated downregulation of LIN-12/Notch upon Ras activation in Caenorhabditis elegans. Nature 420, 686–690 (2002).
Liou, W., Geuze, H.J. Slot, J.W. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 106, 41–58 (1996).
Acknowledgements
We thank H. Fares, Y. Zhang, D. Hirsh and G. Seydoux for important reagents; R. Y. Tsien for mRFP plasmids; S. Mitani for the rabx-5 knockout strain; S. Yamashiro and F. Matsumura for help with mammalian cell culture; and I. Greenwald and H. Fares for critical reading of this manuscript. We also thank P. Schweinsberg, L. Pedraza and W. Przylecki for expert technical assistance. B.D.G. is especially grateful to D. Hirsh for his strong support during the early phases of this work. M.S. and K.S. were supported by JSPS Postdoctoral Fellowship for Research Abroad and Bioarchitect Research Projects of RIKEN, respectively. This work was supported by a NIH Grant GM67237 and MOD Grant 5-FY02-252 to B.D.G. B.D.G. also received support from the Chicago Community Trust Searle Scholars Program. W.L. was supported by a Grant NSC 91-2320-B-182-034 of National Science Council of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary figures S1, S2, S3 and S4 (PDF 423 kb)
Rights and permissions
About this article
Cite this article
Sato, M., Sato, K., Fonarev, P. et al. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat Cell Biol 7, 559–569 (2005). https://doi.org/10.1038/ncb1261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1261
This article is cited by
-
CRISPR-mediated gene targeting of CK1δ/ε leads to enhanced understanding of their role in endocytosis via phosphoregulation of GAPVD1
Scientific Reports (2020)
-
The Rab5 activator RME-6 is required for amyloid precursor protein endocytosis depending on the YTSI motif
Cellular and Molecular Life Sciences (2020)
-
SPIN90, an adaptor protein, alters the proximity between Rab5 and Gapex5 and facilitates Rab5 activation during EGF endocytosis
Experimental & Molecular Medicine (2019)
-
The autophagy receptor ALLO-1 and the IKKE-1 kinase control clearance of paternal mitochondria in Caenorhabditis elegans
Nature Cell Biology (2018)
-
Functional analyses of the plant-specific C-terminal region of VPS9a: the activating factor for RAB5 in Arabidopsis thaliana
Journal of Plant Research (2016)