Abstract
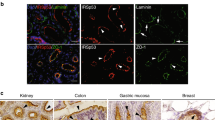
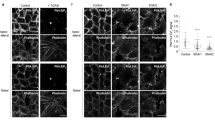
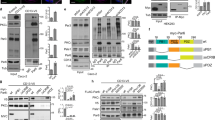
Polarity is a central feature of eukaryotic cells and phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) has a central role in the polarization of neurons and chemotaxing cells. In polarized epithelial cells, PtdIns(3,4,5)P3 is stably localized at the basolateral plasma membrane, but excluded from the apical plasma membrane, as shown by localization of GFP fused to the PtdIns(3,4,5)P3-binding pleckstrin-homology domain of Akt (GFP-PH–Akt), a fusion protein that indicates the location of PtdIns(3,4,5)P3. Here, we ectopically inserted exogenous PtdIns(3,4,5)P3 into the apical plasma membrane of polarized Madin-Darby canine kidney (MDCK) cells. Within 5 min many cells formed protrusions that extended above the apical surface. These protrusions contained basolateral plasma membrane proteins and excluded apical proteins, indicating that their plasma membrane was transformed from apical to basolateral. Addition of PtdIns(3,4,5)P3 to the basolateral surface of MDCK cells grown as cysts caused basolateral protrusions. MDCK cells grown in the presence of a phosphatidylinositol 3-kinase inhibitor had abnormally short lateral surfaces, indicating that PtdIns(3,4,5)P3 regulates the formation of the basolateral surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gibson, M. C. & Perrimon, N. Apicobasal polarization: epithelial form and function. Curr. Opin. Cell Biol. 15, 747–752 (2003).
Nelson, W. J. Adaptation of core mechanisms to generate cell polarity. Nature 422, 766–774 (2003).
Yu, W. et al. β1-integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell 16, 433–445 (2005).
Wodarz, A. Establishing cell polarity in development. Nature Cell Biol. 4, E39–E44 (2002).
Ohno, S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13, 641–648 (2001).
Macara, I. G. Par proteins: partners in polarization. Curr. Biol. 14, R160–R162 (2004).
Mostov, K., Su, T. & ter Beest, M. Polarized epithelial membrane traffic: conservation and plasticity. Nature Cell Biol. 5, 287–293 (2003).
Xu, J. et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214 (2003).
Merlot, S. & Firtel, R. A. Leading the way: Directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 116, 3471–3478 (2003).
Van Haastert, P. J. & Devreotes, P. N. Chemotaxis: signalling the way forward. Nature Rev. Mol. Cell Biol. 5, 626–634 (2004).
Huang, Y. E. et al. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell 14, 1913–1922 (2003).
Meili, R. & Firtel, R. A. Two poles and a compass. Cell 114, 153–156 (2003).
Watton, S. J. & Downward, J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9, 433–436 (1999).
Yu, W. et al. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial sructures. Mol. Biol. Cell 14, 748–763 (2003).
Wang, F. et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nature Cell Biol. 4, 513–518 (2002).
Weiner, O. D. et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nature Cell. Biol. 4, 509–513 (2002).
Ozaki, S., DeWald, D. B., Shope, J. C., Chen, J. & Prestwich, G. D. Intracellular delivery of phosphoinositides and inositol phosphates using polyamine carriers. Proc. Natl Acad. Sci. USA 97, 11286–11291 (2000).
Tian, W., Laffafian, I., Dewitt, S. & Hallett, M. B. Exclusion of exogenous phosphatidylinositol-3,4,5-trisphosphate from neutrophil-polarizing pseudopodia: stabilization of the uropod and cell polarity. EMBO Rep. 4, 982–988 (2003).
Weiner, O. D. et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nature Cell Biol. 4, 509–513 (2002).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Ridley, A. J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477 (2001).
Altschuler, Y. et al. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 143, 1871–1881 (1998).
Mostov, K., Apodaca, G., Aroeti, B. & Okamoto, C. Plasma membrane protein sorting in polarized epithelial cells. J. Cell Biol. 116, 577–583 (1992).
von Stein, W., Ramrath, A., Grimm, A., Muller-Borg, M. & Wodarz, A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development 132, 1675–1686 (2005).
Kierbel, A., Gassama-Diagne, A., Mostov, K. & Engel, J. N. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell 16, 2577–2585 (2005).
Low, S.-H. et al. Differential localization of syntaxin isoforms in polarized MDCK cells. Mol. Biol. Cell 7, 2007–2018 (1996).
Debnath, J., Muthuswamy, S. K. & Brugge, J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003).
Apodaca, G., Katz, L. A. & Mostov, K. E. Receptor-mediated transcytosis of IgA in MDCK cells via apical recycling endosomes. J. Cell Biol. 125, 67–86 (1994).
Hansen, S. H. et al. Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf–MEK-extracellular signal-regulated kinase pathway. Mol. Cell. Biol. 20, 9364–9375 (2000).
Lipschutz, J. H. et al. in Current Protocols Cell in Biology (Wiley and Sons.) 15.5 (2001).
Acknowledgements
We thank K. Matlin, G. Ojakian and B. Stevenson for reagents. We thank H. Bourne, O. Weiner, M. Zegers, P. Brakeman and members of our laboratory for advice and comments on the paper. and D. Mills for help with the manuscript. This work was supported by National Institutes of Health (NIH) grants to K.M. and J.E. W.Y. is supported by a fellowship from the National Kidney Foundation. F.M.-B. is supported by the Human Frontiers Science Program (HFSP).
Author information
Authors and Affiliations
Contributions
A.G. designed, performed and interpreted all of the experiments. W.Y. helped with the design of some experiments. M.t.B. and F.M.-B. made invaluable reagents. A.K. and J.E. helped with the interpretation of data and writing of the paper. K.M. conceived the experiments, helped with interpretation of data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and S4 (PDF 944 kb)
Rights and permissions
About this article
Cite this article
Gassama-Diagne, A., Yu, W., ter Beest, M. et al. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8, 963–970 (2006). https://doi.org/10.1038/ncb1461
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1461
This article is cited by
-
OsSNDP3 Functions for the Polar Tip Growth in Rice Pollen Together with OsSNDP2, a Paralog of OsSNDP3
Rice (2022)
-
Apical–basal polarity and the control of epithelial form and function
Nature Reviews Molecular Cell Biology (2022)
-
PI(4,5)P2 controls slit diaphragm formation and endocytosis in Drosophila nephrocytes
Cellular and Molecular Life Sciences (2022)
-
Phosphatidylinositol 4-kinase IIIβ mediates contraction-induced GLUT4 translocation and shows its anti-diabetic action in cardiomyocytes
Cellular and Molecular Life Sciences (2021)
-
Tools of the trade: studying actin in zebrafish
Histochemistry and Cell Biology (2020)