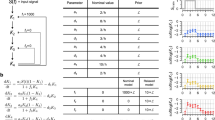
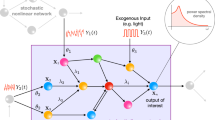
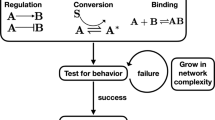
Abstract
Physicochemical modelling of signal transduction links fundamental chemical and physical principles, prior knowledge about regulatory pathways, and experimental data of various types to create powerful tools for formalizing and extending traditional molecular and cellular biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nurse, P. A long twentieth century of the cell cycle and beyond. Cell 100, 71–78 (2000).
Papin, J. A., Price, N. D., Wiback, S. J., Fell, D. A. & Palsson, B. O. Metabolic pathways in the post-genome era. Trends Biochem. Sci. 28, 250–258 (2003).
Reed, J. L., Vo, T. D., Schilling, C. H. & Palsson, B. O. An expanded genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR). Genome Biol. 4, R54 (2003).
Bhalla, U. S., Ram, P. T. & Iyengar, R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 297, 1018–1023 (2002).
Hoffmann, A., Levchenko, A., Scott, M. L. & Baltimore, D. The IκB–NF-κB signaling module: temporal control and selective gene activation. Science 298, 1241–1245 (2002).
Huang, C. Y. & Ferrell, J. E., Jr. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA 93, 10078–10083 (1996).
Markevich, N. I., Hoek, J. B. & Kholodenko, B. N. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J. Cell Biol. 164, 353–359 (2004).
Schoeberl, B., Eichler-Jonsson, C., Gilles, E. D. & Muller, G. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nature Biotechnol. 20, 370–375 (2002).
Oda, K., Matsuoka, Y., Funahashi, A. & Kitano, H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 1, 0010 (2005).
Gardiner, C. W. Handbook of Stochastic Processes (Springer, New York, 2005).
Danuser, G. & Waterman-Storer, C. M. Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 35, 361–387 (2006).
Mallavarapu, A. & Mitchison, T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J. Cell Biol. 146, 1097–1106 (1999).
Odde, D. J. & Buettner, H. M. Time series characterization of simulated microtubule dynamics in the nerve growth cone. Ann. Biomed. Eng. 23, 268–286 (1995).
Ponti, A., Machacek, M., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782–1786 (2004).
McAdams, H. H. & Arkin, A. Stochastic mechanisms in gene expression. Proc. Natl Acad. Sci. USA 94, 814–819 (1997).
Paulsson, J. Summing up the noise in gene networks. Nature 427, 415–418 (2004).
Conzelmann, H., Saez-Rodriguez, J., Sauter, T., Kholodenko, B. N. & Gilles, E. D. A domain-oriented approach to the reduction of combinatorial complexity in signal transduction networks. BMC Bioinformatics 7, 34 (2006).
Blinov, M. L., Faeder, J. R., Goldstein, B. & Hlavacek, W. S. A network model of early events in epidermal growth factor receptor signaling that accounts for combinatorial complexity. Biosystems 83, 136–151 (2006).
Hlavacek, W. S. et al. Rules for modeling signal-transduction systems. Sci. STKE re6 (2006).
Tolle, D. P. & Le Novere, N. Particle-Based Stochastic Simulation in Systems Biology. Current Bioinformatics 1, 1–6 (2006).
Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Tyson, J. J., Chen, K. C. & Novak, B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15, 221–231 (2003).
von Dassow, G., Meir, E., Munro, E. M. & Odell, G. M. The segment polarity network is a robust developmental module. Nature 406, 188–192 (2000).
Conrad, E. D. & Tyson, J. J. in System Modeling in Cellular Biology (eds. Szallasi, Z., Stelling, J. & Periwal, V.) 97–123 (MIT Press, Cambridge, 2006).
Farrow, L. A. & Edelson, D. The steady-state assumption: fact or fiction? Int. J. Chem. Kin. 1, 309–322 (1974).
Flach, E. H. & Schnell, S. Use and abuse of the quasi-steady-state approximation. IEE Proc. Syst. Biol. 153, 187–191 (2006).
Segel, L. A. On the validity of the steady state assumption of enzyme kinetics. Bull. Math. Biol. 50, 579–593 (1988).
Balci, O. in Proceedings of the 29th conference on Winter simulation 135–141 (ACH Press, Atlanta, 1997).
Sargent, R. G. in 2005 Proceedings of the Winter Simulation Conference 14 (ACH Press, New York, 2005).
van Riel, N. A. W. & Sontag, E. D. Parametric estimation in models combining signal transduction and metabolic pathways: the dependent input approach. IEE Proc.Syst. Biol. 153, 263–274 (2006).
Geva-Zatorsky, N. et al. Oscillations and variability in the p53 system. Mol. Syst. Biol. 2, 0033 (2006).
Aldridge, B. B., Haller, G., Sorger, P. K. & Lauffenburger, D. A. Direct Lyaponov exponent analysis enables parametric study of transient signalling governing cell behaviour. IEE Proc. Syst. Biol. 153, (2006).
Bentele, M. et al. Mathematical modeling reveals threshold mechanism in CD95-induced apoptosis. J. Cell Biol. 166, 839–851 (2004).
Frey, D. & Li, X. in Engineering Systems 2004 Symposium (MIT Engineering Systems Division, Cambridge, 2004).
Wiggins, S. in Introduction to Applied Nonlinear Dynamical Systems and Chaos (eds. Marsden, J. E., Sirovich, L. & Antman, S. S.) 356–xxx (Springer-Verlag, New York, 2003).
Hoppenstaedt, F. C. Analysis and Simulation of Chaotic Systems (Springer-Verlag, New York, 2000).
Hucka, M. et al. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics 19, 524–531 (2003).
Merks, R. M. H. & Glazier, J. A. A cell-centered approach to developmental biology. Physica A 352, 113–130 (2005).
Dyson, F. A meeting with Enrico Fermi. Nature 427, 297 (2004).
Kitano, H., Funahashi, A., Matsuoka, Y. & Oda, K. Using process diagrams for the graphical representation of biological networks. Nature Biotechnol. 23, 961–966 (2005).
Moles, C. G., Mendes, P. & Banga, J. R. Parameter estimation in biochemical pathways: a comparison of global optimization methods. Genome Res. 13, 2467–2474 (2003).
Alves, R., Antunes, F. & Salvador, A. Tools for kinetic modeling of biochemical networks. Nature Biotechnol. 24, 667–672 (2006).
Le Novere, N. et al. Minimum information requested in the annotation of biochemical models (MIRIAM). Nature Biotechnol 23, 1509–1515 (2005).
Gillespie, D. T. A Rigorous Derivation of the Chemical Master Equation. Physica A 188, 404–425 (1992).
Roussel, M. R. & Zhu, R. Reducing a chemical master equation by invariant manifold methods. J. Chem. Phys. 121, 8716–8730 (2004).
Acknowledgements
We thank G. Danuser, J. Gunawardena, B. Schoeberl and W. Fontana for critical reading of this manuscript. This work was funded by a National Institutes of Health (NIH) grant P50-GM68762 and a Deparment of Energy (DOE) Computational Science Graduate Fellowship to B.B.A. (DE-FG02-97ER25308).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Aldridge, B., Burke, J., Lauffenburger, D. et al. Physicochemical modelling of cell signalling pathways. Nat Cell Biol 8, 1195–1203 (2006). https://doi.org/10.1038/ncb1497
Issue Date:
DOI: https://doi.org/10.1038/ncb1497
This article is cited by
-
Physiological significance of bistable circuit design in metabolic homeostasis: role of integrated insulin-glucagon signalling network
Molecular Biology Reports (2022)
-
Using optimal control to understand complex metabolic pathways
BMC Bioinformatics (2020)
-
Knowledge-primed neural networks enable biologically interpretable deep learning on single-cell sequencing data
Genome Biology (2020)
-
LogicNet: probabilistic continuous logics in reconstructing gene regulatory networks
BMC Bioinformatics (2020)
-
Feedback analysis identifies a combination target for overcoming adaptive resistance to targeted cancer therapy
Oncogene (2020)