Abstract
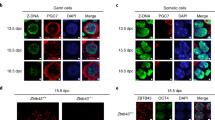
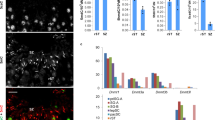
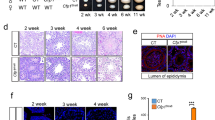
Lymphoid specific helicase (Lsh) is a major epigenetic regulator that is essential for DNA methylation and transcriptional silencing of parasitic elements in the mammalian genome1,2. However, whether Lsh is involved in the regulation of chromatin-mediated processes during meiosis is not known. Here, we show that Lsh is essential for the completion of meiosis and transcriptional repression of repetitive elements in the female gonad. Oocytes from Lsh knockout mice exhibit demethylation of transposable elements and tandem repeats at pericentric heterochromatin, as well as incomplete chromosome synapsis associated with persistent RAD51 foci and γH2AX phosphorylation. Failure to load crossover-associated foci results in the generation of non-exchange chromosomes. The severe oocyte loss observed and lack of ovarian follicle formation, together with the patterns of Lsh nuclear compartmentalization in the germ line, demonstrate that Lsh has a critical and previously unidentified role in epigenetic gene silencing and maintenance of genomic stability during female meiosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Huang, J. et al. Lsh, an epigenetic guardian of repetitive elements. Nucleic Acids Res. 32, 5019–5028 (2004).
Muegge, K. Lsh, a guardian of heterochromatin at repeat elements. Biochem. Cell Biol. 83, 548–554 (2005).
Jarvis, C. et al. A novel putative helicase produced in early murine lymphocytes. Gene 169, 203–207 (1996).
Geiman, T., Durum, S. & Muegge, K. Characterization of gene expression, genomic structure, and chromosomal localization of Hells (Lsh). Genomics 54, 477–483 (1998).
Sun, L. et al. Growth retardation and premature aging phenotypes in mice with disruption of the SNF2-like gene, PASG. Genes Dev. 18, 1035–1046 (2004).
Geiman, T. et al. Lsh, a SNF2 family member, is required for normal murine development. Biochim. Biophys. Acta. 1526, 211–220 (2001).
Dennis, K. et al. Lsh, a member of the SNF2 family, is required for genome-wide methylation. Genes Dev. 15, 2940–2944 (2001).
Yan, Q. et al. Lsh, a modulator of CpG methylation, is crucial for normal histone methylation. EMBO J. 22, 5154–5162 (2003).
Fan, T. et al. Lsh controls silencing of the imprinted Cdkn1c gene. Development. 132, 635–644 (2005).
Reik, W., Dean, W. & Walter, J. Epigenetic reprogramming in mammalian development. Science 293, 1089–1093 (2001).
Page, S. & Hawley, R. Chromosome choreography: the meiotic ballet. Science 301, 785–789 (2003).
Mahadevaiah, S. K. et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nature Genet. 27, 271–276 (2001).
Petukhova, G., Romanienko, P. & Camerini-Otero, R. The Hop2 protein has a direct role in promoting interhomolog interactions during mouse meiosis. Dev. Cell 5, 927–936 (2003).
Peters, A. H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Bourc'his, D. & Bestor, T. H. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 431, 96–99 (2004).
Webster, K. E. et al. Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis. Proc. Natl Acad. Sci. USA 102, 4068–4073 (2005).
Hassold, T. & Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nature Rev. Genet. 2, 280–291 (2001).
Johnson, J. et al. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428, 145–150 (2004).
Obata Y, Kono, T. & Hatada, I. Maturation of mouse fetal germ cells in vitro. Nature 418, 497 (2002).
Lammers, J. et al. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol. 14, 1137–1146 (1994).
Turner, J. et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nature Genet. 37, 41–47 (2005).
Pittman, D. et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1, 697–705 (1998).
Baker, S. M. et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nature Genet. 13, 336–342 (1996).
Hayashi, K., Yoshida, K. & Matsui, Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438, 374–378 (2005).
Di Giacomo, M. et al. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc. Natl Acad. Sci USA 102, 737–742 (2005).
Zhu, H. et al. Lsh is involved in de novo methylation of DNA. EMBO J. 25, 335–345 (2006).
Kneitz, B. et al. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 14, 1085–1097 (2000).
Libby, B. J. et al. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev. Biol. 242, 174–187 (2002).
Yuan, L. et al. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 296, 115–118 (2002).
De La Fuente, R. et al. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev. Biol. 275, 447–458 (2004).
Acknowledgements
We thank C. Heyting, C. Hoog, R. Benavente and B. Earnshaw for generous gifts of antibodies, and M. M. Viveiros for helpful comments during manuscript preparation. This research was supported by a grant from the National Institute of Child Health and Human Development (NICHD) National Institutes of Health (NIH; HD042740) to R.D.L.F. and by NIH grant (RR17359) to I.D. This project has been funded in part with Federal funds from the National Cancer Institute (NCI), NIH, under Contract No. N01-C0-12400 to K.M. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
This study was designed, overseen and written by R.D.L.F. Analyses of gene expression and DNA methylation were conducted by C.B. Analysis of meiotic configurations and germ-cell culture were conducted by R.D.L.F. T.F. and A.S. conducted the sample collection and genotyping. I.D. contributed to project planning and a critical review of the manuscript. K.M. generated the transgenic mouse strain and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 (PDF 477 kb)
Rights and permissions
About this article
Cite this article
De La Fuente, R., Baumann, C., Fan, T. et al. Lsh is required for meiotic chromosome synapsis and retrotransposon silencing in female germ cells. Nat Cell Biol 8, 1448–1454 (2006). https://doi.org/10.1038/ncb1513
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1513
This article is cited by
-
Lymphoid-specific helicase in epigenetics, DNA repair and cancer
British Journal of Cancer (2022)
-
LSH mediates gene repression through macroH2A deposition
Nature Communications (2020)
-
Helicase LSH/Hells regulates kinetochore function, histone H3/Thr3 phosphorylation and centromere transcription during oocyte meiosis
Nature Communications (2020)
-
High expression of Endogenous Retroviruses from intrauterine life to adulthood in two mouse models of Autism Spectrum Disorders
Scientific Reports (2018)
-
Lsh/HELLS regulates self-renewal/proliferation of neural stem/progenitor cells
Scientific Reports (2017)