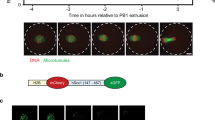
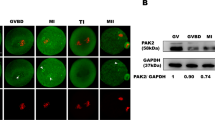
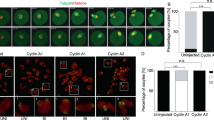
Abstract
The first female meiotic division (meiosis I, MI) is uniquely prone to chromosome segregation errors through non-disjunction, resulting in trisomies and early pregnancy loss1. Here, we show a fundamental difference in the control of mammalian meiosis that may underlie such susceptibility. It involves a reversal in the well-established timing of activation of the anaphase-promoting complex (APC)2,3 by its co-activators cdc20 and cdh1. APCcdh1 was active first, during prometaphase I, and was needed in order to allow homologue congression, as loss of cdh1 speeded up MI, leading to premature chromosome segregation and a non-disjunction phenotype. APCcdh1 targeted cdc20 for degradation, but did not target securin or cyclin B1. These were degraded later in MI through APCcdc20, making cdc20 re-synthesis essential for successful meiotic progression. The switch from APCcdh1 to APCcdc20 activity was controlled by increasing CDK1 and cdh1 loss. These findings demonstrate a fundamentally different mechanism of control for the first meiotic division in mammalian oocytes that is not observed in meioses of other species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev. Genet. 2, 280–291 (2001).
Acquaviva, C. & Pines, J. The anaphase-promoting complex/cyclosome: APC/C. J. Cell Sci. 119, 2401–2404 (2006).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006).
Bashir, T., Dorrello, N. V., Amador, V., Guardavaccaro, D. & Pagano, M. Control of the SCFSkp2-Cks1 ubiquitin ligase by the APC/CCdh1 ubiquitin ligase. Nature 428, 190–193 (2004).
Wei, W. et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 (2004).
Pines, J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 (2006).
Reis, A., Chang, H. Y., Levasseur, M. & Jones, K. T. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nature Cell Biol. 8, 539–540 (2006).
Marangos, P., Verschuren, E. W., Chen, R., Jackson, P. K. & Carroll, J. Prophase I arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APCCdh1. J. Cell Biol. 176, 65–75 (2007).
Homer, H. A. et al. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 19, 202–207 (2005).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Herbert, M. et al. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nature Cell Biol. 5, 1023–1025 (2003).
Hampl, A. & Eppig, J. J. Analysis of the mechanism(s) of metaphase I arrest in maturing mouse oocytes. Development 121, 925–933 (1995).
Vodermaier, H. C., Gieffers, C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13, 1459–1468 (2003).
Rape, M., Reddy, S. K. & Kirschner, M. W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 (2006).
Binne, U. K. et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nature Cell Biol. 9, 225–232 (2007).
Wan, Y., Liu, X. & Kirschner, M. W. The anaphase-promoting complex mediates TGF-β signaling by targeting SnoN for destruction. Mol. Cell 8, 1027–1039 (2001).
Jeganathan, K. B., Malureanu, L. & van Deursen, J. M. The Rae1-Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature 438, 1036–1039 (2005).
Salah, S. M. & Nasmyth, K. Destruction of the securin Pds1p occurs at the onset of anaphase during both meiotic divisions in yeast. Chromosoma 109, 27–34 (2000).
Taieb, F. E., Gross, S. D., Lewellyn, A. L. & Maller, J. L. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr. Biol. 11, 508–513 (2001).
Peter, M. et al. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nature Cell Biol. 3, 83–87 (2001).
Lee, B. H. & Amon, A. Role of Polo-like kinase CDC5 in programming meiosis I chromosome segregation. Science 300, 482–486 (2003).
Kitagawa, R., Law, E., Tang, L. & Rose, A. M. The Cdc20 homolog, FZY-1, and its interacting protein, IFY-1, are required for proper chromosome segregation in Caenorhabditis elegans. Curr. Biol. 12, 2118–2123 (2002).
Zachariae, W., Schwab, M., Nasmyth, K. & Seufert, W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282, 1721–1724 (1998).
Listovsky, T., Zor, A., Laronne, A. & Brandeis, M. Cdk1 is essential for mammalian cyclosome/APC regulation. Exp. Cell Res. 255, 184–191 (2000).
Listovsky, T. et al. Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 23, 1619–1626 (2004).
Benmaamar, R. & Pagano, M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle 4, 1230–1232 (2005).
Brunet, S., Pahlavan, G., Taylor, S. & Maro, B. Functionality of the spindle checkpoint during the first meiotic division of mammalian oocytes. Reproduction 126, 443–450 (2003).
Wassmann, K., Niault, T. & Maro, B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr. Biol. 13, 1596–1608 (2003).
LeMaire-Adkins, R., Radke, K. & Hunt, P. A. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J. Cell Biol. 139, 1611–1619 (1997).
Gorr, I. H. et al. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nature Cell Biol. 8, 1035–1037 (2006).
Madgwick, S., Hansen, D. V., Levasseur, M., Jackson, P. K. & Jones, K. T. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J. Cell Biol. 174, 791–801 (2006).
Acknowledgements
We thank the technical support of Olaf Stemmann (Max Planck Institute of Biochemistry, Munich, Germany) in performing the H1 kinase assays. This work was supported by a grant from the Wellcome Trust to K.T.J.
Author information
Authors and Affiliations
Contributions
K.T.J. directed the work. A.R. and S.M. performed most of the experiments, with H.-Y.C making the initial observations on the effects of the cdh1MO, I.N. performing some of the western blots and M.L. making some of the constructs. K.T.J. wrote the paper in consultation with A.R. and S.M.
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 (PDF 240 kb)
Supplementary Information
Supplementary Movie 1 (AVI 2851 kb)
Supplementary Information
Supplementary Movie 2 (AVI 2456 kb)
Rights and permissions
About this article
Cite this article
Reis, A., Madgwick, S., Chang, HY. et al. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol 9, 1192–1198 (2007). https://doi.org/10.1038/ncb1640
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1640
This article is cited by
-
A prometaphase mechanism of securin destruction is essential for meiotic progression in mouse oocytes
Nature Communications (2021)
-
Phosphorylation of the Anaphase Promoting Complex activator FZR1/CDH1 is required for Meiosis II entry in mouse male germ cell
Scientific Reports (2020)
-
Biallelic mutations in CDC20 cause female infertility characterized by abnormalities in oocyte maturation and early embryonic development
Protein & Cell (2020)
-
Cdk1 inactivation induces post-anaphase-onset spindle migration and membrane protrusion required for extreme asymmetry in mouse oocytes
Nature Communications (2018)
-
The Translation of Cyclin B1 and B2 is Differentially Regulated during Mouse Oocyte Reentry into the Meiotic Cell Cycle
Scientific Reports (2017)