Abstract
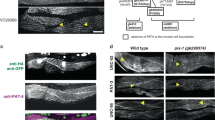
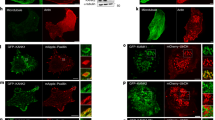
Integrin transmembrane receptors mediate cell adhesion through intracellular linker proteins that connect to the cytoskeleton1,2. Of the numerous linker proteins identified, only a few, including Talin and Integrin-linked-kinase (ILK), are essential and evolutionarily conserved. The wech gene encodes a newly discovered and highly conserved regulator of integrin-mediated adhesion in Drosophila melanogaster. Embryos deficient in wech have very similar phenotypes to integrin-null or Talin-null embryos, including muscle detachment from the body wall. The Wech protein contains a B-box zinc-finger and a coiled-coil domain, which is also found in RBCC/TRIM family3 members, and an NHL domain4. In β-integrin or Talin mutants, Wech is mislocalized, whereas ILK localization depends on Wech. We provide evidence that Wech interacts with the head domain of Talin and the kinase domain of ILK, and propose that Wech is required to connect both core proteins of the linker complex during embryonic muscle attachment. Both the NHL and the B-box/coiled-coil domains of Wech are required for proper interaction with Talin and ILK. The single murine Wech orthologue is also colocalized with Talin and ILK in muscle tissue. We propose that Wech proteins are crucial and evolutionarily conserved regulators of the integrin–cytoskeleton link.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Wiesner, S., Legate, K. R. & Fassler R. Integrin-actin interactions. Cell. Mol. Life Sci. 62, 1081–1099 (2005).
Meroni, G. & Diez-Roux, G. TRIM/RBCC, a novel class of 'single protein RING finger' E3 ubiquitin ligases. Bioessay 27, 1147–1157 (2005).
Slack, F. J. & Ruvkun, G. A novel repeat domain that is often associated with RING finger and B-box motifs. Trends Biochem. Sci. 23, 474–475 (1998).
Gumbiner, B. M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357 (1996).
Brown, N. H., Gregory, S. L. & Martin-Bermudo, M. D. Integrins as mediators of morphogenesis in Drosophila. Dev. Biol. 223, 1–16 (2000).
De Arcangelis, A. & Georges-Labouesse, E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 16, 389–395 (2000).
Giancotti, F. G. & Ruoslahti, E. Integrin signaling. Science 285, 1028–1032 (1999).
Hannigan, G., Troussard, A. A. & Dedhar, S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nature Rev. Cancer 5, 51–63 (2005).
Liu, S., Calderwood, D. A. & Ginsberg, M. H. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113, 3563–3571 (2000).
Wu, C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 15, 460–466 (2005).
Brown, N. H. et al. Talin is essential for integrin function in Drosophila. Dev. Cell 3, 569–579 (2002).
Zervas, C. G., Gregory, S. L. & Brown, N. H. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007–1018 (2001).
Clark, K. A., McGrail, M. & Beckerle, M. C. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130, 2611–2621 (2003).
Torgler, C. N. et al. Tensin stabilizes integrin adhesive contacts in Drosophila. Dev. Cell 6, 357–369 (2004).
Jiang, G., Giannone, G., Critchley, D. R., Fukumoto, E. & Sheetz, M. P. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424, 334–337 (2003).
Tanentzapf, G. & Brown, N. H. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nature Cell Biol. 8, 601–606 (2006).
Giannone, G., Jiang, G., Sutton, D. H., Critchley, D. R. & Sheetz, M. P. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol. 163, 409–419 (2003).
Volk, T. Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 15, 448–453 (1999).
Bokel, C. & Brown, N. H. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 3, 311–321 (2002).
Gregory, S. L. & Brown, N. H. Kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 143, 1271–1282 (1998).
Paululat, A., Breuer, S. & Renkawitz-Pohl, R. Determination and development of the larval muscle pattern in Drosophila melanogaster. Cell Tissue Res. 296, 151–160 (1999).
Leptin, M., Bogaert, T., Lehmann, R. & Wilcox, M. The function of PS integrins during Drosophila embryogenesis. Cell 56, 401–408 (1989).
Roote, C. E. & Zusman, S. Functions for PS integrins in tissue adhesion, migration, and shape changes during early embryonic development in Drosophila. Dev. Biol. 169, 322–336 (1995).
Betschinger, J., Mechtler, K. & Knoblich, J. A. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124, 1241–1253 (2006).
Arama, E., Dickman, D., Kimchie, Z., Shearn, A. & Lev, Z. Mutations in the β-propeller domain of the Drosophila brain tumor (brat) protein induce neoplasm in the larval brain. Oncogene 19, 3706–3716 (2000).
Slack, F. J. et al. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol. Cell 5, 659–669 (2000).
Bendig, G. et al. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 20, 2361–2372 (2006).
Mayer, U. Integrins: redundant or important players in skeletal muscles? J. Biol. Chem. 278, 14587–14590 (2003).
Acknowledgements
We would like to thank N. Brown and D. Kiehart for sharing fly stocks and reagents, A. Bill for measuring the intensity of the Wech interactions, D. Fürst for discussion of the murine muscle data, T. Magin, I. Zinke and P. Carrera for comments on the manuscript and the members of the Hoch laboratory for helpful discussions. This work was supported by grants from the Deutsche Forschungsgemeinschaft to M. H. and W. K. (SFB 645).
Author information
Authors and Affiliations
Contributions
B. L. performed all the Drosophila experiments, which were designed together with R. B. and M. H.; W. K. designed the mouse experiments; J. N. characterized the murine Wech 5B7 antibody which was produced by E. K.; R. Bo. performed the sectioning and staining of the murine muscles. All authors discussed the experimental results of the manuscript. M.H. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5 and Supplementary Methods (PDF 1142 kb)
Rights and permissions
About this article
Cite this article
Löer, B., Bauer, R., Bornheim, R. et al. The NHL-domain protein Wech is crucial for the integrin–cytoskeleton link. Nat Cell Biol 10, 422–428 (2008). https://doi.org/10.1038/ncb1704
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1704
This article is cited by
-
Evolutionary plasticity of the NHL domain underlies distinct solutions to RNA recognition
Nature Communications (2018)
-
Environmental sensing through focal adhesions
Nature Reviews Molecular Cell Biology (2009)
-
The mitotic functions of integrin-linked kinase
Cancer and Metastasis Reviews (2009)