Abstract
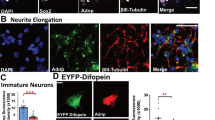
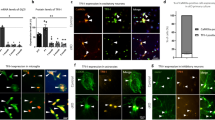
Mitochondrial morphology is dynamically controlled by a balance between fusion and fission. The physiological importance of mitochondrial fission in vertebrates is less clearly defined than that of mitochondrial fusion. Here we show that mice lacking the mitochondrial fission GTPase Drp1 have developmental abnormalities, particularly in the forebrain, and die after embryonic day 12.5. Neural cell-specific (NS) Drp1−/− mice die shortly after birth as a result of brain hypoplasia with apoptosis. Primary culture of NS-Drp1−/− mouse forebrain showed a decreased number of neurites and defective synapse formation, thought to be due to aggregated mitochondria that failed to distribute properly within the cell processes. These defects were reflected by abnormal forebrain development and highlight the importance of Drp1-dependent mitochondrial fission within highly polarized cells such as neurons. Moreover, Drp1−/− murine embryonic fibroblasts and embryonic stem cells revealed that Drp1 is required for a normal rate of cytochrome c release and caspase activation during apoptosis, although mitochondrial outer membrane permeabilization, as examined by the release of Smac/Diablo and Tim8a, may occur independently of Drp1 activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Okamoto, K. & Shaw, J. M. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 39, 503–536 (2005).
Chan, D. C. Mitochondrial fusion and fission in mammals. Annu. Rev. Dev. Biol. 22, 79–99 (2006).
McBride, H. M., Neuspiel, M. & Wasiak, S. Mitochondria: more than a powerhouse. Curr. Biol. 16, R551–R560 (2006).
Gandre-Babbe, S. & van der Bliek, A. M. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 19, 2402–2412 (2008).
Delettre, C. et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nature Genet. 26, 207–210 (2000).
Alexander, C. et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature Genet. 26, 211–215 (2000).
Zuchner, S. et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nature Genet. 36, 449–451 (2004).
Chen, H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 (2003).
Chen H., McCaffery, J. M. & Chan, D. C. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130, 548–562 (2007).
Frank, S. et al. The role of dynamin-related protein 1, a mediator mitochondrial fission, in apoptosis. Dev Cell. 1, 515–525 (2001).
Lee, Y. J., Jeong, S. Y., Karbowski, M., Smith, C. L. & Youle, R. J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15, 5001–5011 (2004).
Suen, D.-F., Norris, K. L. & Youle, R. J. Mitochondrial dynamics and apoptosis. Genes Dev. 22, 1577–1590 (2008).
Twig, G. et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 (2008).
Labrousse, A. M., Zappaterra, M. D., Rube, D. A. & van der Bliek, A. M. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell 4, 815–826 (1999).
Li, Z., Okamoto, K., Hayashi, Y. & Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887 (2004).
Verstreken, P. et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365–378 (2005).
Arnoult, D. Mitochondrial fragmentation in apoptosis. Trends Cell Biol. 17, 6–12 (2007).
Martinou, J.-C. & Youle, R. J. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ. 13, 1291–1295 (2006).
Parone, P. A. et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol. Cell. Biol. 26, 7397–7408 (2006).
Waterham, H. R. et al. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 356, 1736–1741 (2007).
Koch, A. et al. Dynamin-like protein 1 is involved in peroxisomal fission. J. Biol. Chem. 278, 8597–8605 (2003).
Ishihara, N., Jofuku, A., Eura, Y. & Mihara, K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 301, 891–898 (2003).
Ishihara, N., Fujita, Y., Oka, T. & Mihara, K. Regulation of mitochondrial morphology through proteolysic cleavage of OPA1. EMBO J. 25, 2966–2977 (2006).
Cassidy-Stone, A. et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell 14, 193–204 (2008).
Parone, P. A. et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3, e3257 (2009).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Benard, G. et al. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 120, 838–848 (2007).
Taguchi, N., Ishihara, N., Jofuku, A., Oka, T. & Mihara, K. Mitochondrial phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 282, 11521–11529 (2007).
Koehler, C. M. New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20, 309–335 (2004).
Arnoult, D., Grodet, A., Lee, Y.-J., Estaquier, J. & Blackstone, C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 280, 35742–35750 (2005).
Zimmerman, L. et al. Independent regulatory elements in the Nestin gene direct trangene expression to neural stem cells or muscle precursors. Neuron 12, 11–24 (1994).
Luo, L. & O'Leary, D. D. M. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 (2005).
Uesaka, N., Hayano, Y., Yamada, A. & Yamamoto, N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J. Neurosci. 27, 5215–23 (2007).
Koch, A., Schneider, G., Lüers, G. H. & Schrader, M. Peroxisome elongation and constriction but not fission can occur independently of dynamin-like protein 1. J. Cell Sci. 117, 3995–4006 (2004).
Chang, D. T. W. & Reynolds, I. J. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog. Neurobiol. 80, 242–268 (2006).
Li, H. et al. Bcl-XL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc. Natl Acad. Sci. USA 105, 2169–2174 (2008).
Szabadkai, G. et al. Drp1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16, 59–68 (2004).
Uren, R. T. et al. Mitochondrial release of pro-apoptogenic proteins. Electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 280, 2266–2274 (2005).
Frezza, C. et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 (2006).
Sun, M. G. et al. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nature Cell Biol. 9, 1057–1065 (2007).
Yamaguchi, R. et al. OPA1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol. Cell 31, 1–13 (2008).
Ott, M., Robertson, J. D., Gogvadze, V., Zhivotovsky, B. & Orrenius, S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl Acad. Sci. USA 99, 1259–1263 (2002).
Koppenol, W. H., Vroonland, C. A. & Braams, R. The electric potential field around cytochrome c and the effect of ionic strength on reaction rates of horse cytochrome c. Biochim. Biophys. Acta 503, 499–508 (1978).
Oka, T. et al. Identification of a novel protein MICS1 that is involved in maintenance of mitochondrial morphology and apoptotic release of cytochrome c. Mol. Biol. Cell 19, 2597–2608 (2008).
Wasiak, S., Zunino, R. & McBride, H. M. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol. 177, 439–450 (2007).
Karbowski, M., Norris, K., Cleland, M., Jeong, S. & Youle, R. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658–662 (2006).
Baes, M. et al. A mouse model for Zellweger syndrome. Nature Genet. 17, 49–57 (1997).
Li, X. et al. PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol. Cell. Biol. 22, 4358–4365 (2002).
Ishihara, N., Jofuku, A., Eura, Y. & Mihara, K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 301, 891–898 (2003).
Kato, H., Sakaki, K. & Mihara, K. Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J. Cell Sci. 119, 2342–2353 (2006).
Suzuki, H., Okazawa, Y., Komiya, T. & Mihara, K. Characterization of rat Tom40, a central cmponent of the preprotein translocase of the mitochondrial outer membrane. J. Biol. Chem. 275, 37930–37936 (2000).
Jofuku, A., Ishihara, N. & Mihara, K. Analysis of the functional domains of rat mitochondrial Fis1, the mitchondrial fission-stimulating protein. Biochem. Biophys. Res. Commun. 333, 650–659 (2005).
Ishihara, N. & Mihara, K. Identification of the protein import components of the rat mitochondrial inner membrane, rTIM17, rTIM23 and rTIM44. J. Biochem. (Tokyo) 123, 722–732 (1998).
Eura, Y., Ishihara, N., Yokota, S. & Mihara, K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J. Biochem. (Tokyo) 134, 333–344 (2003).
Seligman, A. M., Karnovsky, M. J., Wasserkrug, H. L. & Hanker, J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmophilic reagent, diaminobenzidine (DAB). J. Cell Biol. 38, 1–14 (1968).
Acknowledgements
We thank Dr Toshihiko Oka, Dr Noboru Mizushima, Dr Akira Kondo and members of the Mihara laboratory for productive discussions. We also thank Dr Richard Youle and Dr Atsushi Tanaka for critical reading of the manuscript and for advice. This work was supported by grants from the Ministry of Education, Science, and Culture of Japan, from the Human Frontier Science Program, from Core Research from Evolutional Science and Technology, and from the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
M.N., A.J., K. Masuda, N.I., Y.N. and H.M. contributed to the generation of Drp1−/− mice and NS-Drp1−/− mice, and S.O.S., A.J. and H.K. analysed the phenotypes mainly in the brain. S.O.S. performed the diagnoses on the mice brain. S.O.S., A.J., N.I. and H.K. contributed to analyses of neuronal primary cultured cells of NS-Drp1−/− mice. N.I., A.J., N.T. and M.M. contributed to analyses of Drp1−/− ES and MEF cells, and H.O. and A.J. analysed the apoptotic response. I.N. and Y.G. contributed to histochemical EM for Drp1−/− mice brain, and Y.S. contributed to all conventional EM analyses. K. Mihara planned the project, analysed the data, and wrote the manuscript except the part relating to medical diagnosis for the brains of Drp1-knockout mice, which was written by S.O.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2174 kb)
Rights and permissions
About this article
Cite this article
Ishihara, N., Nomura, M., Jofuku, A. et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11, 958–966 (2009). https://doi.org/10.1038/ncb1907
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1907
This article is cited by
-
Cdk8/CDK19 promotes mitochondrial fission through Drp1 phosphorylation and can phenotypically suppress pink1 deficiency in Drosophila
Nature Communications (2024)
-
Functional, structural, and molecular remodelling of the goldfish (Carassius auratus) heart under moderate hypoxia
Fish Physiology and Biochemistry (2024)
-
Amyloid-β accumulation in human astrocytes induces mitochondrial disruption and changed energy metabolism
Journal of Neuroinflammation (2023)
-
SETD5 haploinsufficiency affects mitochondrial compartment in neural cells
Molecular Autism (2023)
-
Enhanced primary ciliogenesis via mitochondrial oxidative stress activates AKT to prevent neurotoxicity in HSPA9/mortalin-depleted SH-SY5Y cells
Molecular Brain (2023)