Abstract
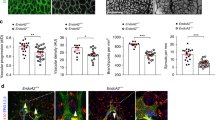
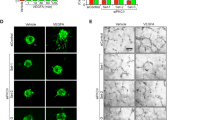
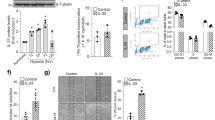
Slit–Roundabout (Robo) signalling has a well-understood role in axon guidance1,2,3,4,5. Unlike in the nervous system, however, Slit-dependent activation of an endothelial-specific Robo, Robo4, does not initiate a guidance program. Instead, Robo4 maintains the barrier function of the mature vascular network by inhibiting neovascular tuft formation and endothelial hyperpermeability induced by pro-angiogenic factors6. In this study, we used cell biological and biochemical techniques to elucidate the molecular mechanism underlying the maintenance of vascular stability by Robo4. Here, we demonstrate that Robo4 mediates Slit2-dependent suppression of cellular protrusive activity through direct interaction with the intracellular adaptor protein paxillin and its paralogue, Hic-5. Formation of a Robo4–paxillin complex at the cell surface blocks activation of the small GTPase Arf6 and, consequently, Rac by recruitment of Arf-GAPs (ADP-ribosylation factor- directed GTPase-activating proteins) such as GIT1. Consistent with these in vitro studies, inhibition of Arf6 activity in vivo phenocopies Robo4 activation by reducing pathologic angiogenesis in choroidal and retinal vascular disease and VEGF-165 (vascular endothelial growth factor-165)-induced retinal hyperpermeability. These data reveal that a Slit2–Robo4–paxillin–GIT1 network inhibits the cellular protrusive activity underlying neovascularization and vascular leak, and identify a new therapeutic target for ameliorating diseases involving the vascular system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Seeger, M., Tear, G., Ferres-Marco, D. & Goodman, C. S. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron 10, 409–426 (1993).
Brose, K. et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806 (1999).
Kidd, T., Bland, K. S. & Goodman, C. S. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785–794 (1999).
Kidd, T. et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92, 205–215 (1998).
Li, H. S. et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96, 807–818 (1999).
Jones, C. A. et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature Med. 14, 448–453 (2008).
Park, K. W. et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev. Biol. 261, 251–267 (2003).
Seth, P. et al. Magic roundabout, a tumor endothelial marker: expression and signaling. Biochem. Biophys. Res. Comm. 332, 533–541 (2005).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Turner, C. E. Paxillin and focal adhesion signalling. Nature cell Biol. 2, E231–236 (2000).
Yuminamochi, T. et al. Expression of the LIM proteins paxillin and Hic-5 in human tissues. J. Histochem. Cytochem. 51, 513–521 (2003).
Hohenester, E., Hussain, S. & Howitt, J. A. Interaction of the guidance molecule Slit with cellular receptors. Biochem. Soc. Trans. 34, 418–421 (2006).
Jones, C. A. et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature Med. (2008).
Bashaw, G. J., Kidd, T., Murray, D., Pawson, T. & Goodman, C. S. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703–715 (2000).
Turner, C. E. Paxillin interactions. J. Cell Sci. 113 (Pt 23), 4139–4140 (2000).
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Nobes, C. D. & Hall, A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244 (1999).
Nishiya, N., Kiosses, W. B., Han, J. & Ginsberg, M. H. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nature Cell Biol. 7, 343–352 (2005).
Zhang, Q. et al. Small-molecule synergist of the Wnt/β-catenin signaling pathway. Proceedings of the National Academy of Sciences of the United States of America 104, 7444–7448 (2007).
Ikeda, S. et al. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 96, 467–475 (2005).
Hafner, M. et al. Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature 444, 941–944 (2006).
Acknowledgements
We thank J. Wythe for critical reading of the manuscript, and D. Lim for expert graphical assistance. J. Bonafacino provided the GST–GGA3 expression plasmid. This work was funded by grants from the H.A. and Edna Benning Foundation, the Juvenile Diabetes Research Foundation, the American Heart Association, the Burroughs Wellcome Fund and the Department of Defense (D.Y.L.); the National Heart Lung and Blood Institute (D.Y.L. and M.H.G.); the National Eye Institute (K.Z.); the Deutsche Forschungsgemeinschaft SFB 704 (M.F.); the National Institute of Arthritis and Musculoskeletal and Skin Diseases (M.H.G.); the National Institute of Allergy and Disease AI065357 (D.Y.L.) and by the US National Institutes of Health, Ruth L. Kirschstein National Research Service Award (N.R.L.) and training grant T32-GM007464 (A.C.C.).
Author information
Authors and Affiliations
Contributions
C.A.J., N.N., N.R.L., M.H.G. and D.Y.L. were responsible for project conceptualization and planning, experimental design, data analysis, and manuscript preparation. M.H.G. and D.Y.L. were responsible for funding the project. C.A.J., N.N. and N.R.L were responsible for performing all experiments or coordinating experimental design and work of others. W.Z., L.K.S., A.C., C.J.L. and K.R.T. performed specific and necessary experiments presented in the paper or in our response to reviewers. Q.Z., P.G.S., A.M.H., M.F. and K.Z. provided expertise, reagents or assays.
Corresponding authors
Ethics declarations
Competing interests
The authors declare competing financial interests. The University of Utah has filed patents covering the technology described in this manuscript with the intent of commercializing this technology.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1328 kb)
Rights and permissions
About this article
Cite this article
Jones, C., Nishiya, N., London, N. et al. Slit2–Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol 11, 1325–1331 (2009). https://doi.org/10.1038/ncb1976
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1976
This article is cited by
-
Proteomic profiling in cerebral amyloid angiopathy reveals an overlap with CADASIL highlighting accumulation of HTRA1 and its substrates
Acta Neuropathologica Communications (2022)
-
Trafficking in blood vessel development
Angiogenesis (2022)
-
Coordination of endothelial cell positioning and fate specification by the epicardium
Nature Communications (2021)
-
Evogliptin, a dipeptidyl peptidase-4 inhibitor, attenuates pathological retinal angiogenesis by suppressing vascular endothelial growth factor-induced Arf6 activation
Experimental & Molecular Medicine (2020)
-
SLIT2/ROBO1-signaling inhibits macropinocytosis by opposing cortical cytoskeletal remodeling
Nature Communications (2020)