Abstract
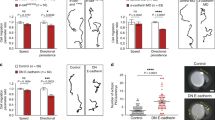
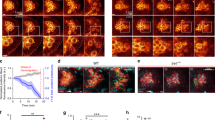
Cell migration is central to embryonic development, homeostasis and disease1, processes in which cells move as part of a group or individually. Whereas the mechanisms controlling single-cell migration in vitro are relatively well understood2,3,4, less is known about the mechanisms promoting the motility of individual cells in vivo. In particular, it is not clear how cells that form blebs in their migration use those protrusions to bring about movement in the context of the three-dimensional cellular environment5,6. Here we show that the motility of chemokine-guided germ cells within the zebrafish embryo requires the function of the small Rho GTPases Rac1 and RhoA, as well as E-cadherin-mediated cell–cell adhesion. Using fluorescence resonance energy transfer we demonstrate that Rac1 and RhoA are activated in the cell front. At this location, Rac1 is responsible for the formation of actin-rich structures, and RhoA promotes retrograde actin flow. We propose that these actin-rich structures undergoing retrograde flow are essential for the generation of E-cadherin-mediated traction forces between the germ cells and the surrounding tissue and are therefore crucial for cell motility in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Horwitz, R. & Webb, D. Cell migration. Curr. Biol. 13, R756–R759 (2003).
Lauffenburger, D. A. & Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Puklin-Faucher, E. & Sheetz, M. P. The mechanical integrin cycle. J. Cell Sci. 122, 179–186 (2009).
Fackler, O. T. & Grosse, R. Cell motility through plasma membrane blebbing. J. Cell Biol. 181, 879–884 (2008).
Charras, G. & Paluch, E. Blebs lead the way: how to migrate without lamellipodia. Nature Rev. Mol. Cell Biol. 9, 730–736 (2008).
Kunwar, P. S., Siekhaus, D. E. & Lehmann, R. In vivo migration: a germ cell perspective. Annu. Rev. Cell Dev. Biol. 22, 237–265 (2006).
Doitsidou, M. et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 111, 647–659 (2002).
Reichman-Fried, M., Minina, S. & Raz, E. Autonomous modes of behavior in primordial germ cell migration. Dev. Cell 6, 589–596 (2004).
Blaser, H. et al. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev. Cell 11, 613–627 (2006).
Boldajipour, B. et al. Control of chemokine-guided cell migration by ligand sequestration. Cell 132, 463–473 (2008).
Heasman, S. J. & Ridley, A. J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature Rev. Mol. Cell Biol. 9, 690–701 (2008).
Jaffe, A. B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Sanders, L. C., Matsumura, F., Bokoch, G. M. & de Lanerolle, P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283, 2083–2085 (1999).
Itoh, R. E. et al. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22, 6582–6591 (2002).
Wong, K., Pertz, O., Hahn, K. & Bourne, H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc. Natl Acad. Sci. USA 103, 3639–3644 (2006).
Sander, E. E., ten Klooster, J. P., van Delft, S., van der Kammen, R. A. & Collard, J. G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009–1022 (1999).
Pertz, O., Hodgson, L., Klemke, R. L. & Hahn, K. M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069–1072 (2006).
Kurokawa, K. & Matsuda, M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol. Biol. Cell 16, 4294–4303 (2005).
Yoshizaki, H. et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 162, 223–232 (2003).
Gupton, S. L. & Waterman-Storer, C. M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 125, 1361–1374 (2006).
Gardel, M. L. et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 183, 999–1005 (2008).
Mould, A. P. et al. Identification of multiple integrin β1 homologs in zebrafish (Danio rerio). BMC Cell Biol. 7, 24 (2006).
Gumbiner, B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Rev. Mol. Cell Biol. 6, 622–634 (2005).
Blaser, H. et al. Transition from non-motile behaviour to directed migration during early PGC development in zebrafish. J. Cell Sci. 118, 4027–4038 (2005).
Mich, J. K. et al. Germ cell migration in zebrafish is cyclopamine-sensitive but Smoothened-independent. Dev. Biol. 328, 342–354 (2009).
Kemler, R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 9, 317–321 (1993).
Nelson, W. J. Regulation of cell–cell adhesion by the cadherin–catenin complex. Biochem. Soc. Trans. 36, 149–155 (2008).
Oyama, T. et al. A truncated β-catenin disrupts the interaction between E-cadherin and α-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res. 54, 6282–6287 (1994).
Kunwar, P. S. et al. Tre1 GPCR initiates germ cell transepithelial migration by regulating Drosophila melanogaster E-cadherin. J. Cell Biol. 183, 157–168 (2008).
Kane, D. A., McFarland, K. N. & Warga, R. M. Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development 132, 1105–1116 (2005).
Lin, F. et al. Gα12/13 regulate epiboly by inhibiting E-cadherin activity and modulating the actin cytoskeleton. J. Cell Biol. 184, 909–921 (2009).
Geisbrecht, E. R. & Montell, D. J. Myosin VI is required for E-cadherin-mediated border cell migration. Nature Cell Biol. 4, 616–620 (2002).
Fulga, T. A. & Rorth, P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nature Cell Biol. 4, 715–719 (2002).
Hogan, C. et al. Characterization of the interface between normal and transformed epithelial cells. Nature Cell Biol. 11, 460–467 (2009).
Wang, S. P. et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nature Cell Biol. 11, 694–704 (2009).
Köprunner, M., Thisse, C., Thisse, B. & Raz, E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 15, 2877–2885 (2001).
Nguyen, A. W. & Daugherty, P. S. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nature Biotechnol. 23, 355–360 (2005).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nature Methods 5, 605–607 (2008).
Daoudi, M. et al. Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J. Biol. Chem. 279, 19649–19657 (2004).
Acknowledgements
The FRET biosensors for Rac and RhoA were a gift from M. Matsuda. The yellow variant of GFP, YPet, was generously provided by P. Daugherty. Lifeact fusions were a gift from R. Wedlich-Soldner, and the GFP-CLIP-170 was provided by Franck Perez. We thank Donna Arndt-Jovin and Tom Jovin for their help with the FRET experiments. This work is supported by grants from the Deutsche Forschungsgemeinschaft (DFG) and the Max Planck Society. E.K. and B.B. are students at the International Max Planck Research School, Göttingen.
Author information
Authors and Affiliations
Contributions
E.K. performed all the experiments except for the following: the initial characterization of Rac and RhoA phenotypes, generation of enhanced green fluorescent protein–actin transgenic line, track analysis in Figs 1, 2 and 4 and Supplementary Information, Fig. S7c, and in situ analysis in Supplementary Information, Fig. S1b were performed by M.R.; cell behaviour in Matrigel was conducted by B.B., adhesion measurements were performed by J.-L.M. and C.-P. H.; and the experiment described in Supplementary Information, Fig. S7a was performed by E.P. and C.-P.H. E.M. cloned DNA constructs and performed RNA injections. E.K., M.R. and E.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1722 kb)
Supplementary Information
Supplementary Movie 1 (MOV 5571 kb)
Supplementary Information
Supplementary Movie 2 (MOV 2590 kb)
Supplementary Information
Supplementary Movie 3 (MOV 2770 kb)
Supplementary Information
Supplementary Movie 4 (MOV 252 kb)
Supplementary Information
Supplementary Movie 5 (MOV 936 kb)
Supplementary Information
Supplementary Movie 6 (MOV 1101 kb)
Supplementary Information
Supplementary Movie 7 (MOV 1827 kb)
Supplementary Information
Supplementary Movie 8 (MOV 4083 kb)
Supplementary Information
Supplementary Movie 9 (MOV 7840 kb)
Supplementary Information
Supplementary Movie 10 (MOV 8964 kb)
Supplementary Information
Supplementary Movie 11 (MOV 8915 kb)
Supplementary Information
Supplementary Movie 12 (MOV 7483 kb)
Supplementary Information
Supplementary Movie 13 (MOV 7177 kb)
Supplementary Information
Supplementary Movie 14 (MOV 8484 kb)
Supplementary Information
Supplementary Movie 15 (MOV 8569 kb)
Rights and permissions
About this article
Cite this article
Kardash, E., Reichman-Fried, M., Maître, JL. et al. A role for Rho GTPases and cell–cell adhesion in single-cell motility in vivo. Nat Cell Biol 12, 47–53 (2010). https://doi.org/10.1038/ncb2003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2003
This article is cited by
-
Zebrafish imaging reveals TP53 mutation switching oncogene-induced senescence from suppressor to driver in primary tumorigenesis
Nature Communications (2022)
-
Micropipette-based biomechanical nanotools on living cells
European Biophysics Journal (2022)
-
E-cadherin focuses protrusion formation at the front of migrating cells by impeding actin flow
Nature Communications (2020)
-
Non-junctional role of Cadherin3 in cell migration and contact inhibition of locomotion via domain-dependent, opposing regulation of Rac1
Scientific Reports (2020)
-
Tools of the trade: studying actin in zebrafish
Histochemistry and Cell Biology (2020)