Abstract
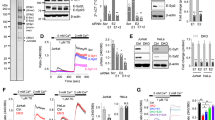
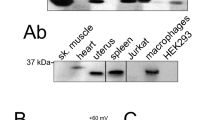
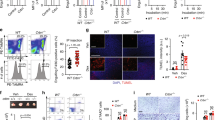
Orai1 and STIM1 are critical components of Ca2+ release-activated Ca2+ (CRAC) channels that mediate store-operated Ca2+ entry (SOCE) in immune cells. Although it is known that Orai1 and STIM1 co-cluster and physically interact to mediate SOCE, the cytoplasmic machinery modulating these functions remains poorly understood. We sought to find modulators of Orai1 and STIM1 using affinity protein purification and identified a novel EF-hand protein, CRACR2A (also called CRAC regulator 2A, EFCAB4B or FLJ33805). We show that CRACR2A interacts directly with Orai1 and STIM1, forming a ternary complex that dissociates at elevated Ca2+ concentrations. Studies using knockdown mediated by small interfering RNA (siRNA) and mutagenesis show that CRACR2A is important for clustering of Orai1 and STIM1 upon store depletion. Expression of an EF-hand mutant of CRACR2A enhanced STIM1 clustering, elevated cytoplasmic Ca2+ and induced cell death, suggesting its active interaction with CRAC channels. These observations implicate CRACR2A, a novel Ca2+ binding protein that is highly expressed in T cells and conserved in vertebrates, as a key regulator of CRAC channel-mediated SOCE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 (2007).
Vig, M. & Kinet, J. P. Calcium signaling in immune cells. Nat. Immunol. 10, 21–27 (2009).
Gwack, Y., Feske, S., Srikanth, S., Hogan, P. G. & Rao, A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 42, 145–156 (2007).
Lewis, R. S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 (2001).
Cahalan, M. D. & Chandy, K. G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 231, 59–87 (2009).
Liou, J. et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 (2005).
Roos, J. et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 (2005).
Zhang, S. L. et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 (2005).
Luik, R. M., Wang, B., Prakriya, M., Wu, M. M. & Lewis, R. S. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454, 538–542 (2008).
Feske, S. et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006).
Prakriya, M. et al. Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 (2006).
Vig, M. et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 16, 2073–2079 (2006).
Vig, M. et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 (2006).
Yeromin, A. V. et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 (2006).
Zhang, S. L. et al. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl Acad. Sci. USA 103, 9357–9362 (2006).
Gwack, Y. et al. Biochemical and functional characterization of Orai proteins. J. Biol. Chem. 282, 16232–16243 (2007).
Luik, R. M., Wu, M. M., Buchanan, J. & Lewis, R. S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174, 815–825 (2006).
Xu, P. et al. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun. 350, 969–976 (2006).
Mercer, J. C. et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 281, 24979–24990 (2006).
Peinelt, C. et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 8, 771–773 (2006).
Soboloff, J. et al. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 281, 20661–20665 (2006).
Muik, M. et al. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J. Biol. Chem. 284, 8421–8426 (2009).
Park, C. Y. et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009).
Yuan, J. P. et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 11, 337–343 (2009).
Muik, M. et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem. 283, 8014–8022 (2008).
Navarro-Borelly, L. et al. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 586, 5383–5401 (2008).
Zhou, Y. et al. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat. Struct. Mol. Biol. 17, 112–116 (2010).
Li, Z. et al. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem. 282, 29448–29456 (2007).
Varnai, P., Toth, B., Toth, D. J., Hunyady, L. & Balla, T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J. Biol. Chem. 282, 29678–29690 (2007).
Liou, J., Fivaz, M., Inoue, T. & Meyer, T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl Acad. Sci. USA 104, 9301–9306 (2007).
Baba, Y. et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 103, 16704–16709 (2006).
Lorenz, H., Hailey, D. W. & Lippincott-Schwartz, J. Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat. Methods 3, 205–210 (2006).
Lorenz, H., Hailey, D. W., Wunder, C. & Lippincott-Schwartz, J. The fluorescence protease protection (FPP) assay to determine protein localization and membrane topology. Nat. Protoc. 1, 276–279 (2006).
Gwack, Y. et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell Biol. 28, 5209–5222 (2008).
Cahalan, M. D. STIMulating store-operated Ca(2+) entry. Nat. Cell Biol. 11, 669–677 (2009).
Putney, J. W. Jr. New molecular players in capacitative Ca2+ entry. J. Cell Sci. 120, 1959–1965 (2007).
Lewis, R. S. The molecular choreography of a store-operated calcium channel. Nature 446, 284–287 (2007).
Baba, Y. & Kurosaki, T. Physiological function and molecular basis of STIM1-mediated calcium entry in immune cells. Immunol. Rev. 231, 174–188 (2009).
Dolmetsch, R. E., Lewis, R. S., Goodnow, C. C. & Healy, J. I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858 (1997).
Dolmetsch, R. E., Xu, K. & Lewis, R. S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 (1998).
Dolmetsch, R. E. & Lewis, R. S. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J. Gen. Physiol. 103, 365–388 (1994).
Mullins, F. M., Park, C. Y., Dolmetsch, R. E. & Lewis, R. S. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc. Natl Acad. Sci. USA 106, 15495–15500 (2009).
Bauer, M. C., O'Connell, D., Cahill, D. J. & Linse, S. Calmodulin binding to the polybasic C-termini of STIM proteins involved in store-operated calcium entry. Biochemistry 47, 6089–6091 (2008).
Nakatani, Y. & Ogryzko, V. Immunoaffinity purification of mammalian protein complexes. Methods Enzymol. 370, 430–444 (2003).
Shi, Y. et al. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422, 735–738 (2003).
Xie, J., Marusich, M. F., Souda, P., Whitelegge, J. & Capaldi, R. A. The mitochondrial inner membrane protein mitofilin exists as a complex with SAM50, metaxins 1 and 2, coiled-coil-helix coiled-coil-helix domain-containing protein 3 and 6 and DnaJC11. FEBS Lett. 581, 3545–3549 (2007).
Srikanth, S., Jung, H. J., Ribalet, B. & Gwack, Y. The intracellular loop of Orai1 plays a central role in fast inactivation of Ca2+ release-activated Ca2+ channels. J. Biol. Chem. 285, 5066–5075 (2010).
Maruyama, K., Mikawa, T. & Ebashi, S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J. Biochem. 95, 511–519 (1984).
Acknowledgements
We thank Kenneth Philipson for critical reading of this manuscript and kind suggestions on Ca2+ overlay experiments. We thank Bernard Ribalet for help with patch clamp experiments, Baljit Khakh for sharing equipments and Earl Homsher for helpful discussions. This work was supported by National Institute of Health grant AI-083432, Stein Oppenheimer Endowment Award, Cancer Research Coordinating Committee Award (Y.G.) and a fellowship from the American Heart Association (S.S.).
Author information
Authors and Affiliations
Contributions
S.S. and Y.G. designed the research. S.S. and Y.G. carried out the experiments with technical help from H.J.J. K.D.K. performed and analysed Ca2+ binding assays and P.S. and J.W. carried out mass spectrometry. S.S. and Y.G. analysed the data and wrote the manuscript. Y.G. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2056 kb)
Rights and permissions
About this article
Cite this article
Srikanth, S., Jung, HJ., Kim, KD. et al. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat Cell Biol 12, 436–446 (2010). https://doi.org/10.1038/ncb2045
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2045
This article is cited by
-
Blockage of Orai1-Nucleolin interaction meditated calcium influx attenuates breast cancer cells growth
Oncogenesis (2022)
-
Affinity-based proteomics reveals novel binding partners for Rab46 in endothelial cells
Scientific Reports (2021)
-
The short isoform of extended synaptotagmin-2 controls Ca2+ dynamics in T cells via interaction with STIM1
Scientific Reports (2020)
-
The orphan solute carrier SLC10A7 is a novel negative regulator of intracellular calcium signaling
Scientific Reports (2020)
-
The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum
Nature Immunology (2019)