Abstract
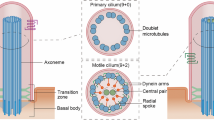
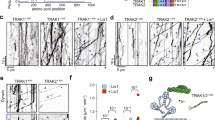
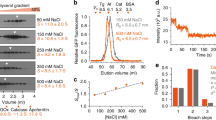
The biogenesis, maintenance and function of primary cilia are controlled through intraflagellar transport (IFT) driven by two kinesin-2 family members, the heterotrimeric KIF3A/KIF3B/KAP complex and the homodimeric KIF17 motor1,2. How these motors and their cargoes gain access to the ciliary compartment is poorly understood. Here, we identify a ciliary localization signal (CLS) in the KIF17 tail domain that is necessary and sufficient for ciliary targeting. Similarities between the CLS and classic nuclear localization signals (NLSs) suggest that similar mechanisms regulate nuclear and ciliary import. We hypothesize that ciliary targeting of KIF17 is regulated by a ciliary-cytoplasmic gradient of the small GTPase Ran, with high levels of GTP-bound Ran (RanGTP) in the cilium. Consistent with this, cytoplasmic expression of GTP-locked Ran(G19V) disrupts the gradient and abolishes ciliary entry of KIF17. Furthermore, KIF17 interacts with the nuclear import protein importin-β2 in a manner dependent on the CLS and inhibited by RanGTP. We propose that Ran has a global role in regulating cellular compartmentalization by controlling the shuttling of cytoplasmic proteins into nuclear and ciliary compartments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Silverman, M. A. & Leroux, M. R. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 19, 306–316 (2009).
Scholey, J. M. Intraflagellar transport motors in cilia: moving along the cell's antenna. J. Cell Biol. 180, 23–29 (2008).
Satir, P., Mitchell, D. R. & Jekely, G. How did the cilium evolve? Curr. Top. Dev. Biol. 85, 63–82 (2008).
Gerdes, J. M., Davis, E. E. & Katsanis, N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45 (2009).
Satir, P. & Christensen, S. T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 (2007).
Scholey, J. M. & Anderson, K. V. Intraflagellar transport and cilium-based signaling. Cell 125, 439–442 (2006).
Tobin, J. L. & Beales, P. L. The nonmotile ciliopathies. Genet. Med. 11, 386–402 (2009).
Fliegauf, M., Benzing, T. & Omran, H. Mechanisms of disease - when cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893 (2007).
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825 (2002).
Ou, G. S., Blacque, O. E., Snow, J. J., Leroux, M. R. & Scholey, J. M. Functional coordination of intraflagellar transport motors. Nature 436, 583–587 (2005).
Snow, J. J. et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6, 1109–1123 (2004).
Insinna, C., Pathak, N., Perkins, B., Drummond, I. & Besharse, J. C. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev. Biol. 316, 160–170 (2008).
Jenkins, P. M. et al. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16, 1211–1216 (2006).
Insinna, C., Humby, M., Sedmak, T., Wolfrum, U. & Besharse, J. C. Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev. Dynam. 238, 2211–2222 (2009).
Gherman, A., Davis, E. E. & Katsanis, N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat. Genet. 38, 961–962 (2006).
Gilula, N. B. & Satir, P. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53, 494–509 (1972).
Luby-Phelps, K., Fogerty, J., Baker, S. A., Pazour, G. J. & Besharse, J. C. Spatial distribution of intraflagellar transport proteins in vertebrate photoreceptors. Vision Res. 48, 413–423 (2008).
Deane, J. A., Cole, D. G., Seeley, E. S., Diener, D. R. & Rosenbaum, J. L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11, 1586–1590 (2001).
Murrell, J. R. & Hunter, D. D. An olfactory sensory neuron line, Odora, properly targets olfactory proteins and responds to odorants. J. Neurosci. 19, 8260–8270 (1999).
Jekely, G. & Arendt, D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays 28, 191–198 (2006).
Devos, D. et al. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLOS biol. 2, 2085–2093 (2004).
Christensen, S. T., Pedersen, L. B., Schneider, L. & Satir, P. Sensory cilia and integration of signal transduction in human health and disease. Traffic 8, 97–109 (2007).
Lee, B. J. et al. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell 126, 543–558 (2006).
Stewart, M. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 8, 195–208 (2007).
Liu, Q. et al. The proteome of the mouse photoreceptor sensory cilium complex. Mol. Cell. Proteomics 6, 1299–1317 (2007).
Richards, S. A., Lounsbury, K. M. & Macara, I. G. The C terminus of the nuclear RAN/TC4 GTPase stabilizes the GDP-bound state and mediates interactions with RCC1, Ran-GAP, and HTF9A/RanBP1. J. Biol. Chem. 270, 14405–14411 (1995).
Lounsbury, K. M., Richards, S. A., Carey, K. L. & Macara, I. G. Mutations within the Ran/TC4 GTPase - Effects on regulatory factor interactions and subcellular localization. J. Biol. Chem. 271, 32834–32841 (1996).
Banaszynski, L. A., Chen, L. C., Maynard-Smith, L. A., Ooi, A. G.L. & Wandless, T. J. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006).
Maynard-Smith, L. A., Chen, L. C., Banaszynski, L. A., Ooi, A. G.L. & Wandless, T. J. A directed approach for engineering conditional protein stability using biologically silent small molecules. J. Biol. Chem. 282, 24866–24872 (2007).
Schoeber, J. P. H. et al. Conditional fast expression and function of multimeric TRPV5 channels using Shield-1. Am. J. Physiol. Renal Physiol. 296, F204–F211 (2009).
Weis, K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112, 441–451 (2003).
Fan, S. L. et al. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J. Cell Biol. 178, 387–398 (2007).
Ems-McClung, S. C., Zheng, Y. X. & Walczak, C. E. Importin alpha/beta and Ran-GTP regulate XCTK2 microtubule binding through a bipartite nuclear localization signal. Mol. Biol. Cell 15, 46–57 (2004).
Tahara, K. et al. Importin-beta and the small guanosine triphosphatase Ran mediate chromosome loading of the human chromokinesin Kid. J. Cell. Biol. 180, 493–506 (2008).
Mazelova, J. et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 28, 183–192 (2009).
Geng, L. et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell. Sci. 119, 1383–1395 (2006).
Pazour, G. J. & Bloodgood, R. A. Targeting proteins to the ciliary membrane. Curr. Top. Dev. Biol. 85, 115–149 (2008).
Hunnicutt, G. R., Kosfiszer, M. G. & Snell, W. J. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii - the cell body plasma-membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J. Cell. Biol. 111, 1605–1616 (1990).
Casanova, J. E. et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47–61 (1999).
Morris, R. L. et al. Redistribution of the kinesin-II subunit KAP from cilia to nuclei during the mitotic and ciliogenic cycles in sea urchin embryos. Dev. Biol. 274, 56–69 (2004).
Cai, D. W., Hoppe, A. D., Swanson, J. A. & Verhey, K. J. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J. Cell. Biol. 176, 51–63 (2007).
Mayer, U. et al. Proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem. Senses 33, 145–162 (2008).
Mayer, U. et al. The proteome of rat olfactory sensory cilia. Proteomics 9, 322–334 (2009).
Davies, S. & Forge, A. Preparation of the mammalian organ of Corti for scanning electron-microscopy. J. Microsc-Oxford 147, 89–101 (1987).
Acknowledgements
This work was supported by NIH grants R01GM070862 and R01GM083254 (to K.J.V.), R01DC009606 (to J.R.M.), R01DK084725 (to B.M.), and T32GM007767 and T32DC00011 (to P.M.J.). Work was also supported by NRSAs F32GM089034 (to J.F.D.) and F31DC009524 (to P.M.J.). H.L.K. is supported as a Barbour Fellow at the University of Michigan. pGEX-Ran plasmids were a kind gift from Brian Burke (University of Florida) and rabbit anti-RanGTP antibody was a kind gift from Ian Macara (University of Virginia).
Author information
Authors and Affiliations
Contributions
J.F.D., H.L.K, P.M.J., S.F. and Y.N.T. performed experiments. J.F.D., H.L.K., P.M.J., J.R.M. and K.J.V. designed experiments. All authors contributed to helpful discussions shaping the aims of the project. J.F.D and K.J.V. wrote the manuscript, with all authors providing detailed comments and suggestions. K.J.V. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1255 kb)
Rights and permissions
About this article
Cite this article
Dishinger, J., Kee, H., Jenkins, P. et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-β2 and RanGTP. Nat Cell Biol 12, 703–710 (2010). https://doi.org/10.1038/ncb2073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2073
This article is cited by
-
Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies
Nature Reviews Nephrology (2024)
-
Cilia regeneration requires an RNA splicing factor from the ciliary base
Cell Regeneration (2022)
-
Molecular Characterization, Tissue Distribution and Localization of Larimichthys crocea Kif3a and Kif3b and Expression Analysis of Their Genes During Spermiogenesis
Journal of Ocean University of China (2019)
-
Kif17 phosphorylation regulates photoreceptor outer segment turnover
BMC Cell Biology (2018)
-
Mechanisms of ciliary targeting: entering importins and Rabs
Cellular and Molecular Life Sciences (2018)