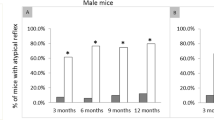
Abstract
Mutations in the tail of the cytoplasmic dynein molecule have been reported to cause neurodegenerative disease in mice. The mutant mouse strain Legs at odd angles (Loa) has impaired retrograde axonal transport, but the molecular deficiencies in the mutant dynein molecule, and how they contribute to neurodegeneration, are unknown. To address these questions, we purified dynein from wild-type mice and the Legs at odd angles mutant mice. Using biochemical, single-molecule, and live-cell-imaging techniques, we find a marked inhibition of motor run-length in vitro and in vivo, and significantly altered motor domain coordination in the dynein from mutant mice. These results suggest a potential role for the dynein tail in motor function, and provide direct evidence for a link between single-motor processivity and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Paschal, B. M. & Vallee, R. B. Retrograde transport by the microtubule associated protein MAP 1C. Nature 330, 181–183 (1987).
Hafezparast, M. et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 300, 808–812 (2003).
Puls, I. et al. Mutant dynactin in motor neuron disease. Nat. Genet. 33, 455–456 (2003).
Lai, C. et al. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J. Neurosci. 27, 13982–13990 (2007).
Chevalier-Larsen, E. S., Wallace, K. E., Pennise, C. R. & Holzbaur, E. L. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum. Mol. Genet. 17, 1946–1955 (2008).
Kieran, D. et al. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J. Cell Biol. 169, 561–567 (2005).
Myers, K. R. et al. Intermediate chain subunit as a probe for cytoplasmic dynein function: biochemical analyses and live-cell imaging in PC12 cells. J. Neurosci. Res. 85, 2640–2647 (2007).
Chen, X. J. et al. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J. Neurosci. 27, 14515–14524 (2007).
Ilieva, H. S. et al. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc. Natl Acad. Sci. USA 105, 12599–12604 (2008).
Perlson, E. et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J. Neurosci. 29, 9903–9917 (2009).
King, S. J., Bonilla, M., Rodgers, M. E. & Schroer, T. A. Subunit organization in cytoplasmic dynein subcomplexes. Protein Sci. 11, 1239–1250 (2002).
Shpetner, H. S., Paschal, B. M. & Vallee, R. B. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP 1C). J. Cell Biol. 107, 1001–1009 (1988).
Ross, J. L., Wallace, K., Shuman, H., Goldman, Y. E. & Holzbaur, E. L. Processive bidirectional motion of dynein–dynactin complexes in vitro. Nat. Cell Biol. 8, 562–570 (2006).
Mallik, R., Carter, B. C., Lex, S. A., King, S. J. & Gross, S. P. Cytoplasmic dynein functions as a gear in response to load. Nature 427, 649–652 (2004).
Reck-Peterson, S. L. et al. Single-molecule analysis of Dynein processivity and stepping behavior. Cell 126, 335–348 (2006).
McKenney, R. J., Vershinin, M., Kunwar, A., Vallee, R. B. & Gross, S. P. LIS1 and NudE induce a persistent dynein force-producing state. Cell 141, 304–314 (2010).
Mallik, R., Petrov, D., Lex, S. A., King, S. J. & Gross, S. P. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr. Biol. 15, 2075–2085 (2005).
King, S. J. & Schroer, T. A. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2, 20–24 (2000).
Kardon, J. R., Reck-Peterson, S. L. & Vale, R. D. Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. Proc. Natl Acad. Sci. USA 106, 5669–5674 (2009).
Zhang, Q. et al. Nudel promotes axonal lysosome clearance and endo-lysosome formation via dynein-mediated transport. Traffic 10, 1337–1349 (2009).
Shubeita, G. T. et al. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 135, 1098–1107 (2008).
Hendricks, A. G. et al. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr. Biol. 20, 697–702 (2010).
Wang, Z., Khan, S. & Sheetz, M. P. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys. J. 69, 2011–2023 (1995).
Shima, T., Imamula, K., Kon, T., Ohkura, R. & Sutoh, K. Head–head coordination is required for the processive motion of cytoplasmic dynein, an AAA+ molecular motor. J. Struct. Biol. 156, 182–189 (2006).
Sweeney, H. L. et al. How myosin VI coordinates its heads during processive movement. EMBO J. 26, 2682–2692 (2007).
Gennerich, A. & Vale, R. D. Walking the walk: how kinesin and dynein coordinate their steps. Curr. Opin. Cell Biol. 21, 59–67 (2009).
Mikami, A., Paschal, B. M., Mazumdar, M. & Vallee, R. B. Molecular cloning of the retrograde transport motor cytoplasmic dynein (MAP 1C). Neuron 10, 787–796 (1993).
Carter, B. C., Shubeita, G. T. & Gross, S. P. Tracking single particles: a user-friendly quantitative evaluation. Phys. Biol. 2, 60–72 (2005).
Vershinin, M., Carter, B. C., Razafsky, D. S., King, S. J. & Gross, S. P. Multiple-motor based transport and its regulation by Tau. Proc. Natl Acad. Sci. USA 104, 87–92 (2007).
Vershinin, M., Xu, J., Razafsky, D. S., King, S. J. & Gross, S. P. Tuning microtubule-based transport through filamentous MAPs: the problem of dynein. Traffic 9, 882–892 (2008).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Asbury, C. L., Shaevitz, J. W., & Lang, M. J. Probing the kinesin reaction cycle with a 2D optical force clamp. Proc. Natl. Acad. Sci. USA 100, 2351-2356 (2003).
Kalafut, B. & Visscher, K. An objective, model-independent method for detection of non-uniform steps in noisy signals. Comput. Phys. Commun. 179, 716–723 (2008).
Kerssemakers, J. W. et al. Assembly dynamics of microtubules at molecular resolution. Nature 442, 709–712 (2006).
Carter, B. C., Vershinin, M. & Gross, S. P. A comparison of step-detection methods: how well can you do? Biophys. J. 94, 306–319 (2008).
Grabham, P. W., Bennecib, M., Seale, G. E., Goldberg, D. J. & Vallee, R. B. Cytoplasmic dynein and LIS1 are required for growth cone remodeling and fast neurite outgrowth. J. Neurosci. 27, 5823–5832 (2007).
Acknowledgements
We thank R. McKenney for help with the quantum dot assays and useful discussion, and M. Vershinnin for developing the tracking program. This work was supported by NIH grants GM47434 (to R.B.V.), RO1GM070676 (to S.P.G.) and GM008798-09 (to K.M.O.M.), AHA grant 825278F (to J.X.), and the Columbia University Motor Neuron Center.
Author information
Authors and Affiliations
Contributions
K.M.O.M., J.X., S.P.G. and R.B.V. designed the research. K.M.O.M. and J.X. performed experiments and analysed data. K.M.O.M., J.X., S.P.G. and R.B.V. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1855 kb)
Rights and permissions
About this article
Cite this article
Ori-McKenney, K., Xu, J., Gross, S. et al. A cytoplasmic dynein tail mutation impairs motor processivity. Nat Cell Biol 12, 1228–1234 (2010). https://doi.org/10.1038/ncb2127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2127
This article is cited by
-
Microtubules gate tau condensation to spatially regulate microtubule functions
Nature Cell Biology (2019)
-
Competition between microtubule-associated proteins directs motor transport
Nature Communications (2018)
-
Step Sizes and Rate Constants of Single-headed Cytoplasmic Dynein Measured with Optical Tweezers
Scientific Reports (2018)
-
Local inhibition of microtubule dynamics by dynein is required for neuronal cargo distribution
Nature Communications (2017)
-
Single Molecule Investigation of Kinesin-1 Motility Using Engineered Microtubule Defects
Scientific Reports (2017)