Abstract
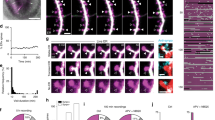
Extension of the endoplasmic reticulum (ER) into dendritic spines of Purkinje neurons is required for cerebellar synaptic plasticity and is disrupted in animals with null mutations in Myo5a, the gene encoding myosin-Va. We show here that myosin-Va acts as a point-to-point organelle transporter to pull ER as cargo into Purkinje neuron spines. Specifically, myosin-Va accumulates at the ER tip as the organelle moves into spines, and hydrolysis of ATP by myosin-Va is required for spine ER targeting. Moreover, myosin-Va is responsible for almost all of the spine ER insertion events. Finally, attenuation of the ability of myosin-Va to move along actin filaments reduces the maximum velocity of ER movement into spines, providing direct evidence that myosin-Va drives ER motility. Thus, we have established that an actin-based motor moves ER within animal cells, and have uncovered the mechanism for ER localization to Purkinje neuron spines, a prerequisite for synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Sellers, J. R. & Weisman, L. S. Myosin V. in Myosins: A Superfamily of Molecular Motors (Springer Netherlands, 2008).
Schott, D. H., Collins, R. N. & Bretscher, A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J. Cell Biol. 156, 35–39 (2002).
Jung, G., Titus, M. A. & Hammer, J. A., 3rd The Dictyostelium type V myosin MyoJ is responsible for the cortical association and motility of contractile vacuole membranes. J. Cell Biol. 186, 555–570 (2009).
Woolner, S. & Bement, W. M. Unconventional myosins acting unconventionally. Trends Cell Biol. 19, 245–252 (2009).
Desnos, C. et al. Myosin Va mediates docking of secretory granules at the plasma membrane. J. Neurosci. 27, 10636–10645 (2007).
Provance, D. W., Jr et al. Myosin-Vb functions as a dynamic tether for peripheral endocytic compartments during transferrin trafficking. BMC Cell Biol. 9, 44 (2008).
Wu, X., Bowers, B., Rao, K., Wei, Q. & Hammer, J. A., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 143, 1899–1918 (1998).
Mercer, J. A., Seperack, P. K., Strobel, M. C., Copeland, N. G. & Jenkins, N. A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 349, 709–713 (1991).
Fukuda, M., Kuroda, T. S. & Mikoshiba, K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 277, 12432–12436 (2002).
Wu, X. S. et al. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 4, 271–278 (2002).
Strom, M., Hume, A. N., Tarafder, A. K., Barkagianni, E. & Seabra, M. C. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 277, 25423–25430 (2002).
Moore, K. J. et al. Dilute suppressor dsu acts semidominantly to suppress the coat color phenotype of a deletion mutation, dl20J, of the murine dilute locus. Proc. Natl Acad. Sci. USA 85, 8131–8135 (1988).
Strobel, M. C., Seperack, P. K., Copeland, N. G. & Jenkins, N. A. Molecular analysis of two mouse dilute locus deletion mutations: spontaneous dilute lethal20J and radiation-induced dilute prenatal lethal Aa2 alleles. Mol. Cell. Biol. 10, 501–509 (1990).
Tilelli, C. Q., Martins, A. R., Larson, R. E. & Garcia-Cairasco, N. Immunohistochemical localization of myosin Va in the adult rat brain. Neuroscience 121, 573–586 (2003).
Miyata, M. et al. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28, 233–244 (2000).
Takagishi, Y. et al. The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci. Lett. 215, 169–172 (1996).
Bourne, J. N. & Harris, K. M. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 31, 47–67 (2008).
English, A. R., Zurek, N. & Voeltz, G. K. Peripheral ER structure and function. Curr. Opin. Cell Biol. 21, 596–602 (2009).
Harris, K. M. & Stevens, J. K. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 8, 4455–4469 (1988).
Matsumoto, M. et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1, 4, 5-trisphosphate receptor. Nature 379, 168–171 (1996).
Street, V. A. et al. The type 1 inositol 1, 4, 5-trisphosphate receptor gene is altered in the opisthotonos mouse. J. Neurosci. 17, 635–645 (1997).
Hartmann, J. & Konnerth, A. Mechanisms of metabotropic glutamate receptor-mediated synaptic signaling in cerebellar Purkinje cells. Acta Physiol. 195, 79–90 (2008).
Kano, M., Hashimoto, K. & Tabata, T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 2173–2186 (2008).
Takechi, H., Eilers, J. & Konnerth, A. A new class of synaptic response involving calcium release in dendritic spines. Nature 396, 757–760 (1998).
Finch, E. A. & Augustine, G. J. Local calcium signalling by inositol-1, 4, 5-trisphosphate in Purkinje cell dendrites. Nature 396, 753–756 (1998).
Bittins, C. M., Eichler, T. W. & Gerdes, H. H. Expression of the dominant-negative tail of myosin Va enhances exocytosis of large dense core vesicles in neurons. Cell. Mol. Neurobiol. 29, 597–608 (2009).
Watanabe, M. et al. Myosin-Va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol. Biol. Cell 16, 4519–4530 (2005).
Xiao, B. et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 21, 707–716 (1998).
Shiraishi-Yamaguchi, Y. & Furuichi, T. The Homer family proteins. Genome Biol. 8, 206 (2007).
Shiraishi, Y., Mizutani, A., Yuasa, S., Mikoshiba, K. & Furuichi, T. Differential expression of Homer family proteins in the developing mouse brain. J. Comp. Neurol. 473, 582–599 (2004).
Jaworski, J. et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61, 85–100 (2009).
Hu, X., Viesselmann, C., Nam, S., Merriam, E. & Dent, E. W. Activity-dependent dynamic microtubule invasion of dendritic spines. J. Neurosci. 28, 13094–13105 (2008).
Bannai, H., Inoue, T., Nakayama, T., Hattori, M. & Mikoshiba, K. Kinesin dependent, rapid, bi-directional transport of ER sub-compartment in dendrites of hippocampal neurons. J. Cell Sci. 117, 163–175 (2004).
Grigoriev, I. et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr. Biol. 18, 177–182 (2008).
Serinagaoglu, Y. et al. A promoter element with enhancer properties, and the orphan nuclear receptor RORalpha, are required for Purkinje cell-specific expression of a Gi/o modulator. Mol. Cell. Neurosci. 34, 324–342 (2007).
Altan-Bonnet, N. et al. Golgi inheritance in mammalian cells is mediated through endoplasmic reticulum export activities. Mol. Biol. Cell 17, 990–1005 (2006).
Petralia, R. S. et al. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. Eur. J. Neurosci. 13, 1722–1732 (2001).
Linden, D. J. & Ahn, S. Activation of presynaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J. Neurosci. 19, 10221–10227 (1999).
Hisatsune, C. et al. Inositol 1, 4, 5-trisphosphate receptor type 1 in granule cells, not in Purkinje cells, regulates the dendritic morphology of Purkinje cells through brain-derived neurotrophic factor production. J. Neurosci. 26, 10916–10924 (2006).
Yengo, C. M., De la Cruz, E. M., Safer, D., Ostap, E. M. & Sweeney, H. L. Kinetic characterization of the weak binding states of myosin V. Biochemistry 41, 8508–8517 (2002).
Shimada, T., Sasaki, N., Ohkura, R. & Sutoh, K. Alanine scanning mutagenesis of the switch I region in the ATPase site of Dictyostelium discoideum myosin II. Biochemistry 36, 14037–14043 (1997).
Walikonis, R. S. et al. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J. Neurosci. 20, 4069–4080 (2000).
Forgacs, E. et al. Switch 1 mutation S217A converts myosin V into a low duty ratio motor. J. Biol. Chem. 284, 2138–2149 (2009).
Sakamoto, T., Yildez, A., Selvin, P. R. & Sellers, J. R. Step-size is determined by neck length in myosin V. Biochemistry 44, 16203–16210 (2005).
Sakamoto, T. et al. Neck length and processivity of myosin V. J. Biol. Chem. 278, 29201–29207 (2003).
Purcell, T. J., Morris, C., Spudich, J. A. & Sweeney, H. L. Role of the lever arm in the processive stepping of myosin V. Proc. Natl Acad. Sci. USA 99, 14159–14164 (2002).
Tabb, J. S., Molyneaux, B. J., Cohen, D. L., Kuznetsov, S. A. & Langford, G. M. Transport of ER vesicles on actin filaments in neurons by myosin V. J. Cell Sci. 111, 3221–3234 (1998).
Wollert, T., Weiss, D. G., Gerdes, H. H. & Kuznetsov, S. A. Activation of myosin V-based motility and F-actin-dependent network formation of endoplasmic reticulum during mitosis. J. Cell Biol. 159, 571–577 (2002).
Estrada, P. et al. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J. Cell Biol. 163, 1255–1266 (2003).
Sparkes, I., Runions, J., Hawes, C. & Griffing, L. Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21, 3937–3949 (2009).
Yokota, E. et al. An isoform of myosin XI is responsible for the translocation of endoplasmic reticulum in tobacco cultured BY-2 cells. J. Exp. Bot. 60, 197–212 (2009).
Ueda, H. et al. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc. Natl Acad. Sci. USA 107, 6894–6899.
Wang, Z. et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 (2008).
Langford, G. M. Actin- and microtubule-dependent organelle motors: interrelationships between the two motility systems. Curr. Opin. Cell Biol. 7, 82–88 (1995).
Toresson, H. & Grant, S. G. Dynamic distribution of endoplasmic reticulum in hippocampal neuron dendritic spines. Eur. J. Neurosci. 22, 1793–1798 (2005).
Holbro, N., Grunditz, A. & Oertner, T. G. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl Acad. Sci. USA 106, 15055–15060 (2009).
Hansel, C. et al. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron 51, 835–843 (2006).
De Zeeuw, C. I. et al. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20, 495–508 (1998).
Seperack, P. K., Mercer, J. A., Strobel, M. C., Copeland, N. G. & Jenkins, N. A. Retroviral sequences located within an intron of the dilute gene alter dilute expression in a tissue-specific manner. EMBO J. 14, 2326–2332 (1995).
Tabata, T. et al. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. J. Neurosci. Methods 104, 45–53 (2000).
Alami, N. H., Jung, P. & Brown, A. Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J. Neurosci. 29, 6625–6634 (2009).
Johnson, H. W. & Schell, M. J. Neuronal IP3 3-kinase is an F-actin bundling protein: role in dendritic targeting and regulation of spine morphology. Mol. Biol. Cell 20, 5166–5180 (2009).
McCroskery, S., Chaudhry, A., Lin, L. & Daniels, M. P. Transmembrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol. Cell. Neurosci. 33, 15–28 (2006).
Acknowledgements
We thank R. Bock and D. J. Linden for teaching us cerebellar culture preparation; J. Oberdick, E. L. Snapp, J. Lippincott-Schwartz, H. D. White, J. R. Sellers, J. A. Martina, S. McCroskery, M. J. Schell and D. S. Bredt for DNA constructs; R. E. Cheney for advice and X. Wu for microscopy support.
Author information
Authors and Affiliations
Contributions
W.W. and J.A.H. designed the project; W.W. carried out the experiments, except the Ca2+ imaging, which was carried out by S.D.B.; J.A.H. contributed new reagents and W.W., S.D.B. and J.A.H. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1635 kb)
Supplementary Movie 1
Supplementary Information (MOV 10581 kb)
Supplementary Movie 2
Supplementary Information (MOV 474 kb)
Supplementary Movie 3
Supplementary Information (MOV 11555 kb)
Supplementary Movie 4
Supplementary Information (MOV 352 kb)
Supplementary Movie 5
Supplementary Information (MOV 4145 kb)
Supplementary Movie 6
Supplementary Information (MOV 1134 kb)
Supplementary Movie 7
Supplementary Information (MOV 12918 kb)
Supplementary Movie 8
Supplementary Information (MOV 12563 kb)
Supplementary Movie 9
Supplementary Information (MOV 12766 kb)
Supplementary Movie 10
Supplementary Information (MOV 5617 kb)
Supplementary Movie 11
Supplementary Information (MOV 1678 kb)
Supplementary Movie 12
Supplementary Information (MOV 521 kb)
Rights and permissions
About this article
Cite this article
Wagner, W., Brenowitz, S. & Hammer, J. Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol 13, 40–48 (2011). https://doi.org/10.1038/ncb2132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2132
This article is cited by
-
Impaired dynamic interaction of axonal endoplasmic reticulum and ribosomes contributes to defective stimulus–response in spinal muscular atrophy
Translational Neurodegeneration (2022)
-
Optical control of fast and processive engineered myosins in vitro and in living cells
Nature Chemical Biology (2021)
-
Endoplasmic reticulum visits highly active spines and prevents runaway potentiation of synapses
Nature Communications (2020)
-
An Improved Method for Differentiating Mouse Embryonic Stem Cells into Cerebellar Purkinje Neurons
The Cerebellum (2019)
-
Multiple myosin motors interact with sodium/potassium-ATPase alpha 1 subunits
Molecular Brain (2018)