Abstract
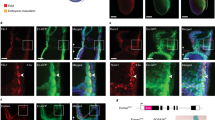
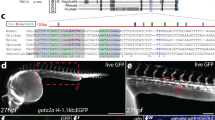
During development, haemogenesis occurs invariably at sites of vasculogenesis. Between embryonic day (E) 9.5 and E10.5 in mice, endothelial cells in the caudal part of the dorsal aorta generate haematopoietic stem cells1,2 and are referred to as haemogenic endothelium3,4,5,6,7,8. The mechanisms by which haematopoiesis is restricted to this domain, and how the morphological transformation from endothelial to haematopoietic is controlled are unknown. We show here that HoxA3, a gene uniquely expressed in the embryonic but not yolk sac vasculature, restrains haematopoietic differentiation of the earliest endothelial progenitors, and induces reversion of the earliest haematopoietic progenitors into CD41-negative endothelial cells. This reversible modulation of endothelial–haematopoietic state is accomplished by targeting key haematopoietic transcription factors for downregulation, including Runx1, Gata1, Gfi1B, Ikaros, and PU.1. Through loss-of-function, and gain-of-function epistasis experiments, and the identification of antipodally regulated targets, we show that among these factors, Runx1 is uniquely able to erase the endothelial program set up by HoxA3. These results suggest both why a frank endothelium does not precede haematopoiesis in the yolk sac, and why haematopoietic stem cell generation requires Runx1 expression only in endothelial cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Godin, I., Dieterlen-Lièvre, F. & Cumano, A. Emergence of multipotent hematopoietic cells in the yolk sac and para-aortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc. Natl Acad. Sci. USA 92, 773–777 (1995).
Muller, A. M., Medvinsky, A., Strouboulis, J., Grosveld, F. & Dzierzak, E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301 (1994).
de Bruijn, M. F. et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16, 673–683 (2002).
North, T. E. et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16, 661–672 (2002).
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E. & Speck, N. A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891 (2009).
Bertrand, J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010).
Boisset, J. C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010).
Kissa, K. & Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010).
McGinnis, W. & Krumlauf, R. Homeobox genes and axial patterning. Cell 68, 283–302 (1992).
Ivanova, N. B. et al. A stem cell molecular signature. Science 298, 601–604 (2002).
Taghon, T. et al. Homeobox gene expression profile in human hematopoietic multipotent stem cells and T-cell progenitors: implications for human T-cell development. Leukemia 17, 1157–1163 (2003).
Forsberg, E. C. et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 1, e28 (2005).
Sauvageau, G. et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc. Natl Acad. Sci. USA 91, 12223–12227 (1994).
Cellot, S. et al. Sustained in vitro trigger of self-renewal divisions in Hoxb4hiPbx1(10) hematopoietic stem cells. Exp. Hematol. 35, 802–816 (2007).
Sauvageau, G. et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 9, 1753–1765 (1995).
Iacovino, M. et al. A conserved role for Hox paralog group 4 in regulation of hematopoietic progenitors. Stem Cells Dev. 18, 783–792 (2009) (2008).
Kyba, M., Perlingeiro, R. C. R. & Daley, G. Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109, 29–37 (2002).
Chisaka, O. & Capecchi, M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature 350, 473–479 (1991).
Myers, C., Charboneau, A. & Boudreau, N. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J. Cell Biol. 148, 343–351 (2000).
Mace, K. A., Hansen, S. L., Myers, C., Young, D. M. & Boudreau, N. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J. Cell Sci. 118, 2567–2577 (2005).
Boudreau, N., Andrews, C., Srebrow, A., Ravanpay, A. & Cheresh, D. A. Induction of the angiogenic phenotype by Hox D3. J. Cell Biol. 139, 257–264 (1997).
Li, W., Ferkowicz, M. J., Johnson, S. A., Shelley, W. C. & Yoder, M. C. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev. 14, 44–54 (2005).
Jaffredo, T., Gautier, R., Eichmann, A. & Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125, 4575–4583 (1998).
North, T. et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575 (1999).
Samokhvalov, I. M., Samokhvalova, N. I. & Nishikawa, S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446, 1056–1061 (2007).
Fehling, H. J. et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130, 4217–4227 (2003).
Choi, K., Kennedy, M., Kazarov, A., Papadimitriou, J. C. & Keller, G. A common precursor for hematopoietic and endothelial cells. Development 125, 725–732 (1998).
Huber, T. L., Kouskoff, V., Fehling, H. J., Palis, J. & Keller, G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432, 625–630 (2004).
Mikkola, H. K., Fujiwara, Y., Schlaeger, T. M., Traver, D. & Orkin, S. H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101, 508–516 (2003).
Mitjavila-Garcia, M. T. et al. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development 129, 2003–2013 (2002).
Lancrin, C. et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895 (2009).
Eilken, H. M., Nishikawa, S. & Schroeder, T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900 (2009).
Yamashita, J. et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92–96 (2000).
Nishikawa, S. I., Nishikawa, S., Hirashima, M., Matsuyoshi, N. & Kodama, H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125, p1747–1757 (1998).
Okuda, T., Deursen, J.v., Hiebert, S. W., Grosveld, G. & Downing, J. R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330 (1996).
Wang, Q. et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl Acad. Sci. USA 93, 3444–3449 (1996).
Bunting, M., Bernstein, K. E., Greer, J. M., Capecchi, M. R. & Thomas, K. R. Targeting genes for self-excision in the germ line. Genes Dev. 13, 1524–1528 (1999).
Sabin, F. R. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib. Embryol. 9, 213–262 (1920).
Guidato, S., Prin, F. & Guthrie, S. Somatic motoneurone specification in the hindbrain: the influence of somite-derived signals, retinoic acid and Hoxa3. Development 130, 2981–2996 (2003).
Xu, K., Chong, D. C., Rankin, S. A., Zorn, A. M. & Cleaver, O. Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev. Biol. (2009).
Acknowledgements
We thank the Bob and Jean Smith Foundation for their generous support. This work was supported by the NIH grant 1R01HL081186-01 and the March of Dimes grant 5-FY2006-272. We thank Nardina Nash for genotyping and animal husbandry.
Author information
Authors and Affiliations
Contributions
M.I., O.C. and M.K. designed the experiments, and wrote the manuscript; M.I. performed the experiments; D.C. and A.C. performed in situ hybridization studies; I.S. performed microarray studies; L.H. and D.R. performed chromatin IP studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 453 kb)
Rights and permissions
About this article
Cite this article
Iacovino, M., Chong, D., Szatmari, I. et al. HoxA3 is an apical regulator of haemogenic endothelium. Nat Cell Biol 13, 72–78 (2011). https://doi.org/10.1038/ncb2137
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2137
This article is cited by
-
Leukocyte-specific DNA methylation biomarkers and their implication for pathological epigenetic analysis
Epigenetics Communications (2022)
-
Dynamic Runx1 chromatin boundaries affect gene expression in hematopoietic development
Nature Communications (2022)
-
Biomechanical cues as master regulators of hematopoietic stem cell fate
Cellular and Molecular Life Sciences (2021)
-
MSX2 suppression through inhibition of TGFβ signaling enhances hematopoietic differentiation of human embryonic stem cells
Stem Cell Research & Therapy (2020)
-
Characterization of a family mutation in the 5’ untranslated region of the endoglin gene causative of hereditary hemorrhagic telangiectasia
Journal of Human Genetics (2019)