Abstract
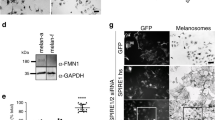
Intracellular transport is vital for the function, survival and architecture of every eukaryotic cell. Long-range transport in animal cells is thought to depend exclusively on microtubule tracks. This study reveals an unexpected actin-dependent but microtubule-independent mechanism for long-range transport of vesicles. Vesicles organize their own actin tracks by recruiting the actin nucleation factors Spire1, Spire2 and Formin-2, which assemble an extensive actin network from the vesicles’ surfaces. The network connects the vesicles with one another and with the plasma membrane. Vesicles move directionally along these connections in a myosin-Vb-dependent manner to converge and to reach the cell surface. The overall outward-directed movement of the vesicle-actin network is driven by recruitment of vesicles to the plasma membrane in the periphery of the oocyte. Being organized in a dynamic vesicle-actin network allows vesicles to move in a local random manner and a global directed manner at the same time: they can reach any position in the cytoplasm, but also move directionally to the cell surface as a collective. Thus, collective movement within a network is a powerful and flexible mode of vesicle transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ross, J. L., Ali, M. Y. & Warshaw, D. M. Cargo transport: Molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 20, 41–47 (2008).
Alberts, B. et al. Molecular Biology of the Cell 5th edn (Garland Science, 2008).
Lodish, H. et al. Molecular Cell Biology (W. H. Freeman, 2007).
Pollard, T. & Earnshaw, W. Cell Biology (Saunders, 2004).
Hirokawa, N., Noda, Y., Tanaka, Y. & Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10, 682–696 (2009).
Kardon, J. R. & Vale, R. D. Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 10, 854–865 (2009).
Bloom, G. S. & Goldstein, L. S. Cruising along microtubule highways: how membranes move through the secretory pathway. J. Cell Biol. 140, 1277–1280 (1998).
Apodaca, G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2, 149–159 (2001).
Fackler, O. T. & Krausslich, H. G. Interactions of human retroviruses with the host cell cytoskeleton. Curr. Opin. Microbiol. 9, 409–415 (2006).
Woolner, S. & Bement, W. M. Unconventional myosins acting unconventionally. Trends Cell Biol. 19, 245–252 (2009).
Wang, Z. et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 (2008).
Wagner, W., Brenowitz, S. D. & Hammer, J. A. 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat. Cell Biol. 13, 40–48 (2011).
Wu, X., Bowers, B., Rao, K., Wei, Q. & Hammer, J. A. 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 143, 1899–1918 (1998).
Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 (2009).
Grant, B. D. & Donaldson, J. G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 (2009).
Schuh, M. & Ellenberg, J. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 (2008).
Soldati, T. & Schliwa, M. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 7, 897–908 (2006).
Schuh, M. & Ellenberg, J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130, 484–498 (2007).
Pfender, S., Kuznetsov, V., Pleiser, S., Kerkhoff, E. & Schuh, M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr. Biol. 21, 955–960 (2011).
Leader, B. et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat. Cell Biol. 4, 921–928 (2002).
Nolen, B. J. et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature 460, 1031–1034 (2009).
Ito, T. et al. Human spire interacts with the barbed end of the actin filament. J. Mol. Biol. 408, 18–25 (2011).
Bosch, M. et al. Analysis of the function of Spire in actin assembly and its synergy with formin and profilin. Mol. Cell. 28, 555–568 (2007).
Campellone, K. G. & Welch, M. D. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11, 237–251 (2010).
Goode, B. L. & Eck, M. J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76, 593–627 (2007).
Lapierre, L. A. et al. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 (2001).
Watanabe, S., Mabuchi, K., Ikebe, R. & Ikebe, M. Mechanoenzymatic characterization of human myosin Vb. Biochemistry 45, 2729–2738 (2006).
Begg, D. A. & Rebhun, L. I. pH regulates the polymerization of actin in the sea urchin egg cortex. J. Cell Biol. 83, 241–248 (1979).
Kerkhoff, E. et al. The Spir actin organizers are involved in vesicle transport processes. Curr. Biol. 11, 1963–1968 (2001).
Gomez, T. S. & Billadeau, D. D. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev. Cell 17, 699–711 (2009).
Morel, E., Parton, R. G. & Gruenberg, J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev. Cell 16, 445–457 (2009).
Galletta, B. J. & Cooper, J. A. Actin and endocytosis: mechanisms and phylogeny. Curr. Opin. Cell Biol. 21, 20–27 (2009).
Woolner, S., O’Brien, L. L., Wiese, C. & Bement, W. M. Myosin-10 and actin filaments are essential for mitotic spindle function. J. Cell Biol. 182, 77–88 (2008).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Dahlgaard, K., Raposo, A. A., Niccoli, T. & St Johnston, D. Capu and Spire assemble a cytoplasmic actin mesh that maintains microtubule organization in the Drosophila oocyte. Dev. Cell 13, 539–553 (2007).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Jaffe, L. A., Norris, R. P., Freudzon, M., Ratzan, W. J. & Mehlmann, L. M. Microinjection of follicle-enclosed mouse oocytes. Methods Mol. Biol. 518, 157–173 (2009).
Eppig, J. J. & Schroeder, A. C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol. Reprod. 41, 268–276 (1989).
Strickland, L. et al. Light microscopy of echinoderm embryos. Methods Cell Biol. 74, 371–409 (2004).
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Sonnichsen, B., De Renzis, S., Nielsen, E., Rietdorf, J. & Zerial, M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914 (2000).
Aschenbrenner, L., Lee, T. & Hasson, T. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol. Biol. Cell 14, 2728–2743 (2003).
Bittins, C. M., Eichler, T. W., Hammer, J. A. 3rd & Gerdes, H. H. Dominant-negative myosin Va impairs retrograde but not anterograde axonal transport of large dense core vesicles. Cell Mol. Neurobiol. 30, 369–379 (2010).
Liman, E. R., Tytgat, J. & Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 (1992).
Burkel, B. M., von Dassow, G. & Bement, W. M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton 64, 822–832 (2007).
Acknowledgements
The author thanks P. Leder, B. Leader and M. Dettenhofer for Fmn2−/− mice; the staff of the LMB’s Animal Facility for expert technical assistance; and M. Freeman, S. Munro and B. Nichols for helpful discussions and critical reading of the manuscript. The research leading to these results has received financial support from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 241548.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1046 kb)
Supplementary movie 1
Supplementary Information (MOV 3272 kb)
Supplementary movie 2
Supplementary Information (MOV 11025 kb)
Supplementary movie 3
Supplementary Information (MOV 8589 kb)
Supplementary movie 4
Supplementary Information (MOV 8356 kb)
Supplementary movie 5
Supplementary Information (MOV 8899 kb)
Supplementary movie 6
Supplementary Information (MOV 3447 kb)
Supplementary movie 7
Supplementary Information (MOV 778 kb)
Supplementary movie 8
Supplementary Information (MOV 8791 kb)
Supplementary movie 9
Supplementary Information (MOV 1880 kb)
Supplementary movie 10
Supplementary Information (MOV 1860 kb)
Rights and permissions
About this article
Cite this article
Schuh, M. An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol 13, 1431–1436 (2011). https://doi.org/10.1038/ncb2353
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2353
This article is cited by
-
Integrated transcriptomics uncovers an enhanced association between the prion protein gene expression and vesicle dynamics signatures in glioblastomas
BMC Cancer (2024)
-
Actin-driven chromosome clustering facilitates fast and complete chromosome capture in mammalian oocytes
Nature Cell Biology (2023)
-
Genetically encoded barcodes for correlative volume electron microscopy
Nature Biotechnology (2023)
-
The nuclear lamina couples mechanical forces to cell fate in the preimplantation embryo via actin organization
Nature Communications (2023)
-
Spire1 and Myosin Vc promote Ca2+-evoked externalization of von Willebrand factor in endothelial cells
Cellular and Molecular Life Sciences (2022)