Abstract
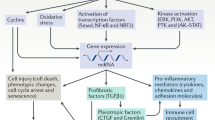
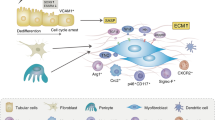
Obstructive nephropathy is a major cause of renal failure, particularly in infants and children. Cellular and molecular mechanisms responsible for the progression of the tubular atrophy and interstitial fibrosis—processes that lead to nephron loss—have been elucidated in the past 5 years. Following urinary tract obstruction and tubular dilatation, a cascade of events results in upregulation of the intrarenal renin–angiotensin system, tubular apoptosis and macrophage infiltration of the interstitium. This is followed by accumulation of interstitial fibroblasts through proliferation of resident fibroblasts and epithelial–mesenchymal transformation of renal tubular cells. Under the influence of cytokines, chemokines and other signaling molecules produced by tubular and interstitial cells, fibroblasts undergo transformation to myofibroblasts that induce expansion of the extracellular matrix. The cellular interactions that regulate development of interstitial inflammation, tubular apoptosis and interstitial fibrosis are complex. Changes in renal gene expression and protein production afford many potential biomarkers of disease progression and targets for therapeutic manipulation. These include signaling molecules and receptors involved in macrophage recruitment and proliferation, tubular death signals and survival factors, and modulators of epithelial–mesenchymal transformation. Targeted gene deletion and various forms of gene therapy have been used in experimental obstructive nephropathy, mostly rodent models of unilateral ureteral obstruction or cell culture techniques. Further refinement of these models is needed to develop a matrix of biomarkers with clinical predictive value, as well as molecular therapies that will prevent or reverse the renal structural and functional consequences of obstructive nephropathy.
Key Points
-
A leading cause of chronic renal failure in children, obstructive nephropathy is characterized by inflammation, tubular atrophy and interstitial fibrosis
-
Research using rodent models in the past 5 years has shed new light on the molecular mechanisms underlying these processes
-
Current biomarkers in congenital obstructive nephropathy are urine levels of tumor-necrosis factor-α, transforming growth factor-β, monocyte chemoattractant protein-1 and epidermal growth factor
-
Potential new biomarkers of disease progression and therapeutic targets include molecules involved in macrophage recruitment and proliferation, tubular death and survival, and epithelial–mesenchymal transformation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Seikaly MG et al. (2003) Chronic renal insufficiency in children: the 2001 annual report of the NAPRTCS. Pediatr Nephrol 18: 796–804
Chevalier RL and Peters CA (2003) Congenital urinary tract obstruction: proceedings of the state-of-the-art strategic planning workshop—National Institutes of Health, Bethesda, Maryland, USA, 11–12 March 2002. Pediatr Nephrol 18: 576–606
Iwano M et al. (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350
Chevalier RL and Cachat F (2001) Role of angiotensin II in chronic ureteral obstruction. In The Renin-Angiotensin System and Progression of Renal Diseases, 250–260 (Ed. Wolf G) Basel: Karger
Ichikawa I et al. (2002) Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int 61: 889–898
Morrissey JJ and Klahr S (1997) Enalapril decreases nuclear factor κB activation in the kidney with ureteral obstruction. Kidney Int 52: 926–933
Klahr S and Morrissey J (2002) Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875
Miyajima A et al. (2003) Novel nuclear factor κB activation inhibitor prevents inflammatory injury in unilateral ureteral obstruction. J Urol 169: 1559–1563
Nakatani T et al. (2002) Role of renin-angiotensin system and nuclear factor-κB in the obstructed kidney of rats with unilateral ureteral obstruction. Jpn J Pharmacol 90: 361–364
Satoh M et al. (2001) Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 12: 317–325
Tashiro K et al. (2003) Attenuation of renal fibrosis by proteasome inhibition in rat obstructive nephropathy: possible role of nuclear factor κB. Int J Mol Med 12: 587–592
Nagatoya K et al. (2002) Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int 61: 1684–1695
Lange-Sperandio B et al. (2002) Selectins mediate macrophage infiltration in obstructive nephropathy in newborn mice. Kidney Int 61: 516–524
Naruse T et al. (2002) P-selectin-dependent macrophage migration into the tubulointerstitium in unilateral ureteral obstruction. Kidney Int 62: 94–105
Ogawa D et al. (2004) Cerebroside sulfotransferase deficiency ameliorates L-selectin-dependent monocyte infiltration in the kidney after ureteral obstruction. J Biol Chem 279: 2085–2090
Takeda A et al. (2000) Role of leukocyte adhesion molecules in monocyte/macrophage infiltration in weanling rats with unilateral ureteral obstruction. Int J Urol 7: 415–420
Lange-Sperandio B et al.: Distinct roles of Mac-1 and its counter-receptors in neonatal obstructive nephropathy. Kidney Int, in press
Cheng QL et al. (2000) Effects of ICAM-1 antisense oligonucleotide on the tubulointerstitium in mice with unilateral ureteral obstruction. Kidney Int 57: 183–190
Yamagishi H et al. (2001) Genetically modified bone marrow-derived vehicle cells site specifically deliver an anti-inflammatory cytokine to inflamed interstitium of obstructive nephropathy. J Immunol 166: 609–616
Vielhauer V et al. (2001) Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol 12: 1173–1187
Wada T et al. (2004) Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol 15: 940–948
Pittock ST et al. (2005) MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622
Lenda DM et al. (2003) Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol 170: 3254–3262
Kitagawa K et al. (2004) Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 165: 237–246
Eis V et al. (2004) Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol 15: 337–347
Anders HJ et al. (2002) A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest 109: 251–259
Lange-Sperandio B et al. (2005) A2A adenosine receptor agonist and PDE4 inhibition delays inflammation but fails to reduce injury in experimental obstructive nephropathy. Nephron Exp Nephrol 100: e113–e123
Schaier M et al. (2003) Retinoid agonist isotretinoin ameliorates obstructive renal injury. J Urol 170: 1398–1402
Hochberg D et al. (2000) Interstitial fibrosis of unilateral ureteral obstruction is exacerbated in kidneys of mice lacking the gene for inducible nitric oxide synthase. Lab Invest 80: 1721–1728
Ito K et al. (2004) Liposome-mediated transfer of nitric oxide synthase gene improves renal function in ureteral obstruction in rats. Kidney Int 66: 1365–1375
Chevalier RL (2004) Promise for gene therapy in obstructive nephropathy. Kidney Int 66: 1709–1710
Ophascharoensuk V et al. (1999) Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int 56: 571–580
Rouschop KMA et al. (2004) CD44 deficiency increases tubular damage but reduces renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 15: 674–686
Zhang G et al. (2003) Urokinase receptor modulates cellular and angiogenic responses in obstructive nephropathy. J Am Soc Nephrol 14: 1234–1253
Nishida M et al. (2002) Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest 110: 1859–1868
Gobe GC and Axelsen RA (1987) Genesis of renal tubular atrophy in experimetal hydronephrosis in the rat. Lab Invest 56: 273–281
Cachat F et al. (2003) Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int 63: 564–575
Miyajima A et al. (2000) Interaction of nitric oxide and transforming growth factor-β1 induced by angiotensin II and mechanical stretch in rat renal tubular epithelial cells. J Urol 164: 1729–1734
Dai C et al. (2003) Transforming growth factor-β1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem 278: 12537–12545
Choi YJ et al. (2001) Role of p53-dependent activation of caspases in chronic obstructive uropathy: evidence from p53 null mutant mice. J Am Soc Nephrol 12: 983–992
Miyajima A et al. (2000) Antibody to transforming growth factor-β ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313
Bhaskaran M et al. (2003) Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol 284: F955–F965
Inguaggiato P et al. (2001) Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int 60: 2181–2191
Power RE et al. (2004) Mechanical deformation induced apoptosis in human proximal renal tubular epithelial cells is caspase dependent. J Urol 171: 457–461
Miyajima A et al. (2001) Role of nitric oxide in renal tubular apoptosis of unilateral ureteral obstruction. Kidney Int 59: 1290–1303
Chevalier RL et al. (1998) Obstructive nephropathy in the neonatal rat is attenuated by epidermal growth factor. Kidney Int 54: 38–47
Chevalier RL et al. (2000) Renal tubulointerstitial injury from ureteral obstruction in the neonatal rat is attenuated by IGF-1. Kidney Int 57: 882–890
Nguyen HT et al. (2000) Heparin-binding EGF-like growth factor is up-regulated in the obstructed kidney in a cell- and region-specific manner and acts to inhibit apoptosis. Am J Pathol 156: 889–898
Chung KH and Chevalier RL (1996) Arrested development of the neonatal kidney following chronic ureteral obstruction. J Urol 155: 1139–1144
Kiley SC et al. (2005) Epidermal growth factor potentiates renal cell death in hydronephrotic neonatal mice, but cell survival in rats. Kidney Int 68: 504–514
Kiley SC et al.: Of mice and men: species differences in epidermal growth factor signaling determine renal cell survival or death. J Am Soc Nephrol, in press
Lange-Sperandio B et al. (2003) Macrophages induce apoptosis in proximal tubule cells. Pediatr Nephrol 18: 335–341
Misseri R et al. (2004) TNF-α mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol 288: F406–F411
Malik RK et al. (2001) Renal apoptosis parallels ceramide content following chronic ureteral obstruction in the neonatal rat. Am J Physiol 281: F56–F61
Basnakian AG et al. (2004) Ceramide synthesis is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. Am J Physiol 288: F308–F314
Sunami R et al. (2004) Acatalasemia sensitizes renal tubular epithelial cells to apoptosis and exacerbates renal fibrosis after unilateral ureteral obstruction. Am J Physiol 286: F1030–F1038
Kinter M et al. (1999) Unilateral ureteral obstruction impairs renal antioxidant enzyme activation during sodium depletion. Kidney Int 55: 1327–1334
Moriyama T et al. (2001) Fluvastatin suppresses oxidative stress and fibrosis in the interstitium of mouse kidneys with unilateral ureteral obstruction. Kidney Int 59: 2095–2103
Pat B et al. (2005) Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney Int 67: 931–943
Kiley SC et al. (2003) Growth factor-mediated phosphorylation of proapoptotic BAD reduces tubule cell death in vitro and in vivo. Kidney Int 63: 33–42
Chevalier RL et al. (2000) Chronic ureteral obstruction in the rat suppresses renal tubular bcl-2 and stimulates apoptosis. Exp Nephrol 8: 115–122
Peherstorfer E et al. (2002) Effects of microinjection of synthetic Bcl-2 domain peptides on apoptosis of renal tubular epithelial cells. Am J Physiol 283: F190–F196
Gao X et al. (2002) Hepatocyte growth factor gene therapy retards the progression of chronic obstructive nephropathy. Kidney Int 62: 1238–1248
Yukawa K et al. (2004) Deletion of the kinase domain in death-associated protein kinase attenuates renal tubular cell apoptosis in chronic obstructive uropathy. Int J Mol Med 13: 515–520
Hughes J and Johnson RJ (1999) Role of Fas (CD95) in tubulointerstitial disease induced by unilateral ureteric obstruction. Am J Physiol 277: F26–F32
Hruska KA et al. (2000) Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol 279: F130–F143
Ophascharoensuk V et al. (1998) The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nat Med 4: 575–580
Schaefer L et al. (2002) Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol 160: 1181–1191
Fern RJ et al. (1999) Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest 103: 39–46
Shin GT et al. (2005) Effects of suppressing intrarenal angiotensinogen on renal transforming growth factor-β1 expression in acute ureteral obstruction. Kidney Int 67: 897–908
Chen CO et al. (2005) Angiotensin converting enzyme inhibition worsens renal interstitial injury from unilateral ureteral obstruction in the neonatal rat. J Am Soc Nephrol 17: 222A
Morrissey JJ et al. (1996) Nitric oxide generation ameliorates the tubulointerstitial fibrosis of obstructive nephropathy. J Am Soc Nephrol 7: 2202–2212
Schanstra JP et al. (2002) In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest 110: 371–379
Fukuda K et al. (2001) Quantification of TGF-β1 mRNA along rat nephron in obstructive nephropathy. Am J Physiol Renal Physiol 281: F513–F521
Kaneto H et al. (1999) Increased expression of TGF-β1 but not of its receptors contributes to human obstructive nephropathy. Kidney Int 56: 2137–2146
Isaka Y et al. (2000) Transforming growth factor-β1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int 58: 1885–1892
Zavadil J et al. (2001) Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proc Natl Acad Sci USA 98: 6686–6691
Fukasawa H et al. (2004) Down-regulation of Smad7 expression by ubiquitin-dependent degradation contributes to renal fibrosis in obstructive nephropathy in mice. Proc Natl Acad Sci USA 101: 8687–8692
Sato M et al. (2003) Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494
Inazaki K et al. (2004) Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int 66: 597–604
Lan HY et al. (2003) Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548
Yang J et al. (2003) Downregulation of Smad transcriptional corepressors SnoN and Ski in the fibrotic kidney: an amplification mechanism for TGF-β1 signaling. J Am Soc Nephrol 14: 3167–3177
Yang J and Liu Y (2002) Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol 13: 96–107
Yang J et al. (2003) Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol 163: 621–632
Yang J et al. (2005) A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol 16: 68–78
Yang J and Liu Y (2003) Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am J Physiol 284: F349–F357
Wamsley-Davis A et al. (2004) AT1A-mediated activation of kidney JNK1 and SMAD2 in obstructive uropathy: preservation of kidney tissue mass using candesartan. Am J Physiol Renal Physiol 287: F474–F480
Yang J et al. (2002) Hepatocyte growth factor gene therapy and angiotensin II blockade synergistically attenuate renal interstitial fibrosis in mice. J Am Soc Nephrol 13: 2464–2477
Nakamura H et al. (2002) Introduction of DNA enzyme for Egr-1 into tubulointerstitial fibroblasts by electroporation reduced interstitial alpha-smooth muscle actin expression and fibrosis in unilateral ureteral obstruction (UUO) rats. Gene Ther 9: 495–502
Taneda S et al. (2003) Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol 14: 2544–2555
Ludewig D et al. (2000) PDGF receptor kinase blocker AG1295 attenuates interstitial fibrosis in rat kidney after unilateral obstruction. Cell Tissue Res 299: 97–103
Yokoi H et al. (2001) Role of connective tissue growth factor in profibrotic action of transforming growth factor-β: a potential target for preventing renal fibrosis. Am J Kidney Dis 38 (Suppl): S134–S138
Yokoi H et al. (2004) Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulinterstitial fibrosis. J Am Soc Nephrol 15: 1430–1440
Stambe C et al. (2004) The role of p38α mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol 15: 370–379
Ishidoya S et al. (2002) Plasminogen activator inhibitor-1 and tissue-type plasminogen activator are up-regulated during unilateral ureteral obstruction in adult rats. J Urol 167: 1503–1507
Oda T et al. (2001) PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596
Matsuo S et al. (2005) Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238
Yang JW et al. (2002) Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest 110: 1525–1538
Zhang G et al. (2005) Urokinase receptor deficiency accelerates renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 14: 1254–1271
Kim HS et al. (2001) TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 12: 736–748
Hughes J et al. (1999) Cyclin kinase inhibitor p21CIP1/WAF1 limits interstitial cell proliferation following ureteric obstruction. Am J Physiol Renal Physiol 277: F948–F956
Morrissey J et al. (2002) Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13: S14–S21
Lin J et al. (2005) Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat Med 11: 387–393
Abreu JG et al. (2002) Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol 4: 599–604
Ingelfinger JR (2003) Forestalling fibrosis. N Engl J Med 349: 2265–2266
Fogo AB (2003) The potential for regression of renal scarring. Curr Opin Nephrol Hypertens 12: 223–225
Chevalier RL (2004) Biomarkers of congenital obstructive nephropathy: past, present and future. J Urol 172: 852–857
Valles P et al. (2003) Role of endogenous nitric oxide in unilateral ureteroplevic junction obstruction in children. Kidney Int 63: 1104–1115
El Sherbiny MT et al. (2002) Role of urinary transforming growth factor-β1 concentration in the diagnosis of upper urinary tract obstruction in children. J Urol 168: 1798–1800
Furness PD III et al. (1999) Elevated bladder urine concentration of transforming growth factor-β1 correlates with upper urinary tract obstruction in children. J Urol 162: 1033–1036
Yang BY et al. (2003) The expression of epidermal growth factor and transforming growth factor-β1 in the stenotic tissue of congenital pelvi-ureteric junction obstruction in children. J Pediatr Surg 38: 1656–1660
Grandaliano G et al. (2000) MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int 58: 182–192
Stephan M et al. (2002) Urinary concentration and tissue messenger RNA expression of monocyte chemoattractant protein-1 as an indicator of the degree of hydronephrotic atrophy in partial ureteral obstruction. J Urol 167: 1497–1502
Chiou YY et al. (2004) Factors associated with the outcomes of children with unilateral ureteropelvic junction obstruction. J Urol 171: 397–402
Seseke F et al. (2004) Characterization of an animal model of spontaneous congenital unilateral obstructive uropathy by cDNA microarray analysis. Eur Urol 45: 374–381
Chevalier RL (2004) Perinatal obstructive nephropathy. Semin Nephrol 28: 124–131
Chevalier RL et al. (1996) Renal apoptosis and clusterin following ureteral obstruction: the role of maturation. J Urol 156: 1474–1479
Chevalier RL et al. (1999) Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int 55: 793–807
Fujinaka H et al. (2000) Salutary role for angiotensin in partial urinary tract obstruction. Kidney Int 58: 2018–2027
Yoo KH et al. (2005) Inducible nitric oxide synthase modulates hydronephrosis following partial or complete unilateral ureteral obstruction in the neonatal mouse. J Am Soc Nephrol 17: 421A
Seseke F et al. (2001) Impaired nephrogenesis in rats with congenital obstructive uropathy. J Urol 165: 2289–2292
Bascands JL and Schanstra JP (2005) Obstructive nephropathy: insights from genetically engineered animals. Kidney Int 68: 925–937
Imai E (2003) Gene therapy for renal diseases: its potential and limitation. J Am Soc Nephrol 14: 1102–1104
Ito K et al. (2004) Liposome-mediated gene therapy in the kidney. Human Cell 17: 17–28
Acknowledgements
The author's studies are supported by grants from the National Institutes of Health: DK52612, DK45179, and DK62328. Photomicrographs (Figure 1) were produced by Michael S Forbes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Chevalier, R. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat Rev Nephrol 2, 157–168 (2006). https://doi.org/10.1038/ncpneph0098
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0098
This article is cited by
-
Glucosidase inhibitor, Nimbidiol ameliorates renal fibrosis and dysfunction in type-1 diabetes
Scientific Reports (2022)
-
Losartan accelerates the repair process of renal fibrosis in UUO mouse after the surgical recanalization by upregulating the expression of Tregs
International Urology and Nephrology (2019)
-
Alpha-lipoic acid ameliorates the epithelial mesenchymal transition induced by unilateral ureteral obstruction in mice
Scientific Reports (2017)
-
Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model
Scientific Reports (2015)
-
Tubular expression of heat-shock protein 27 inhibits fibrogenesis in obstructive nephropathy
Kidney International (2013)