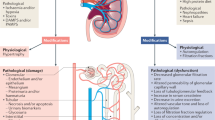
Abstract
Early diagnosis has been the 'Achilles heel' of acute kidney injury (AKI) that has prevented successful implementation of treatment strategies. To date, pharmacological intervention has been largely unsuccessful or equivocal, and morbidity and mortality associated with AKI have remained unacceptably high. Despite their well-known limitations, the most widely used biomarkers for the early diagnosis of AKI are serum creatinine, blood urea nitrogen and urine output. Development of new biomarkers is imperative. A variety of methods have been employed to discover new biomarkers of AKI, including transcriptomics, proteomics, gene arrays, lipidomics and imaging technologies. Clinical trials are underway to establish the validity of the biomarkers discovered using these techniques. This Review summarizes the importance of biomarkers of AKI, from their discovery to clinical practice, from the current perspective and that of what to expect in the future. Great strides forward are being made in breaking down important barriers to the successful prevention and treatment of this devastating disorder.
Key Points
-
A biomarker is a measurable indication of a specific biologic state that is relevant to a specific disease process
-
A perfect biomarker is easily measurable, accurate, reproducible, cost-effective, easy to interpret and provides useful clinical information
-
A biomarker of acute kidney injury (AKI) should independently provide information that is additive to that provided by conventional clinical factors and/or the biomarker should account for a large proportion of the risk associated with AKI
-
Biomarkers would be most useful in AKI for identification of at-risk individuals, surveillance of events that precede the condition, diagnosis and prognosis
-
Numerous potential biomarkers of AKI identified in preclinical investigations are now beginning to be tested in clinical validation studies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hoste EA et al. (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10: R73
Xue JL et al. (2006) Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142
Xue JL et al. (2007) Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306
Hsu CY et al. (2007) Community-based incidence of acute renal failure. Kidney Int 72: 208–212
Chertow GM et al. (2006) Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126
Thakar CV et al. (2005) A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168
Bellomo R et al. (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212
Uchino S et al. (2006) Pulmonary artery catheter versus pulse contour analysis: a prospective epidemiological study. Crit Care 10: R174
Vasan RS (2006) Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 113: 2335–2362
Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89–95
Gutman S and Kessler LG (2006) The US Food and Drug Administration perspective on cancer biomarker development. Nat Rev Cancer 6: 565–571
De Gruttola VG et al. (2001) Considerations in the evaluation of surrogate endpoints in clinical trials: summary of a National Institutes of Health workshop. Control Clin Trials 22: 485–502
Freedman LS et al. (1992) Statistical validation of intermediate endpoints for chronic diseases. Stat Med 11: 167–178
Roberts MA et al. (2006) Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis 48: 341–360
Sargent D and Allegra C (2002) Issues in clinical trial design for tumor marker studies. Semin Oncol 29: 222–230
Gion M et al. (1999) A guide for reviewing submitted manuscripts (and indications for the design of translational research studies on biomarkers). Int J Biol Markers 14: 123–133
Wang TJ et al. (2006) Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 355: 2631–2639
Honda H et al. (2006) Serum albumin, C-reactive protein, interleukin 6, and fetuin A as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148
Diamond GA et al. (1995) Prior restraint: a Bayesian perspective on the optimization of technology utilization for diagnosis of coronary artery disease. Am J Cardiol 76: 82–86
Diamond GA and Kaul S (2004) Prior convictions: Bayesian approaches to the analysis and interpretation of clinical megatrials. J Am Coll Cardiol 43: 1929–1939
Fagan TJ (1975) Letter: nomogram for Bayes theorem. N Engl J Med 293: 257
Justice AC et al. (1999) Assessing the generalizability of prognostic information. Ann Intern Med 130: 515–524
van Houwelingen HC (2000) Validation, calibration, revision and combination of prognostic survival models. Stat Med 19: 3401–3415
Lemeshow S and Hosmer DW Jr (1982) A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 115: 92–106
Liu J et al. (2004) Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA 291: 2591–2599
Ludwig JA and Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 5: 845–856
Morrow DA and Braunwald E (2003) Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation 108: 250–252
Koenig W (2007) Cardiovascular biomarkers: added value with an integrated approach? Circulation 116: 3–5
Hanash S (2003) Disease proteomics. Nature 422: 226–232
Alizadeh AA et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511
Liang Y et al. (2005) Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci USA 102: 5814–5819
Perou CM et al. (2000) Molecular portraits of human breast tumours. Nature 406: 747–752
Sorlie T et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98: 10869–10874
Sotiriou C et al. (2003) Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 100: 10393–10398
van't Veer LJ et al. (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415: 530–536
Knickerbocker T et al. (2007) An integrated approach to prognosis using protein microarrays and nonparametric methods. Mol Syst Biol 3: 123
Rifai N et al. (2006) Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol 24: 971–983
Anderson NL (2005) The roles of multiple proteomic platforms in a pipeline for new diagnostics. Mol Cell Proteomics 4: 1441–1444
Dalton WS and Friend SH (2006) Cancer biomarkers—an invitation to the table. Science 312: 1165–1168
Wilson JF (2006) The rocky road to useful cancer biomarkers. Ann Intern Med 144: 945–948
Hammond ME and Taube SE (2002) Issues and barriers to development of clinically useful tumor markers: a development pathway proposal. Semin Oncol 29: 213–221
Ransohoff DF (2004) Rules of evidence for cancer molecular-marker discovery and validation. Nat Rev Cancer 4: 309–314
HunterM DJ and Kraft P (2007) Drinking from the fire hose—statistical issues in genomewide association studies. N Engl J Med 357: 436–439
Kohane IS et al. (2006) The incidentalome: a threat to genomic medicine. JAMA 296: 212–215
Petricoin EF et al. (2002) Use of proteomic patterns in serum to identify ovarian cancer. Lancet 359: 572–577
Baggerly KA et al. (2005) Signal in noise: evaluating reported reproducibility of serum proteomic tests for ovarian cancer. J Natl Cancer Inst 97: 307–309
Ransohoff DF (2005) Lessons from controversy: ovarian cancer screening and serum proteomics. J Natl Cancer Inst 97: 315–319
Palomba H et al. (2007) Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney Int 72: 624–631
Thakar CV et al. (2005) Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int 67: 1112–1119
Jaber BL et al. (2004) Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine 25: 212–219
Jaber BL et al. (2005) Polymorphism of host response genes: implications in the pathogenesis and treatment of acute renal failure. Kidney Int 67: 14–33
Hirschberg R et al. (1999) Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int 55: 2423–2432
Allgren RL et al. (1997) Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med 336: 828–834
Lassnigg A et al. (2004) Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 15: 1597–1605
Herget-Rosenthal S et al. (2004) Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122
Mehta RL et al. (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31
Akcan-Arikan A et al. (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035
Cruz DN et al. (2007) North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol 2: 418–425
Kuitunen A et al. (2006) Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg 81: 542–546
Mishra J et al. (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238
Wagener G et al. (2006) Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105: 485–491
Bachorzewska-Gajewska H et al. (2006) Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol 26: 287–292
Parikh CR et al. (2005) Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16: 3046–3052
du Cheyron D et al. (2003) Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis 42: 497–506
Thadhani R et al. (1996) Acute renal failure. N Engl J Med 334: 1448–1460
Miller TR et al. (1978) Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 89: 47–50
Espinel CH and Gregory AW (1980) Differential diagnosis of acute renal failure. Clin Nephrol 13: 73–77
Esson ML and Schrier RW (2002) Diagnosis and treatment of acute tubular necrosis. Ann Int Med 137: 744–752
Nguyen MT and Devarajan P (2007) Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol [10.1007/s00467-007-0470-x]
Hewitt SM et al. (2004) Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol 15: 1677–1689
Zhou H et al. (2006) Exosomal fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int 70: 1847–1857
Yamamoto T et al. (2007) Renal L-type fatty acid binding protein in acute ischemic injury. J Am Soc Nephrol 18: 2894–2902
Parikh CR et al. (2006) Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203
Liangos O et al. (2007) Urinary N-acetyl-β-D-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 18: 904–912
Levy H et al. (2005) Steroid use in PROWESS severe sepsis patients treated with drotrecogin alfa (activated). Crit Care 9: R502–R507
Supavekin S et al. (2003) Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63: 1714–1724
Yoshida T et al. (2002) Global analysis of gene expression in renal ischemia-reperfusion in the mouse. Biochem Biophys Res Commun 291: 787–794
Holly MK et al. (2006) Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int 70: 496–506
Nguyen MT et al. (2005) Early prediction of acute renal injury using urinary proteomics. Am J Nephrol 25: 318–326
Portilla D et al. (2006) Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int 69: 2194–2204
Varghese SA et al. (2007) Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol 18: 913–922
Acknowledgements
The authors acknowledge that portions of this manuscript were derived from a meeting held by the Acute Kidney Injury Network in Vancouver, BC, Canada, 11–14 September 2006. This work was supported by NIH grants DK56223, DK62324 and DK58413 to MDO, and by DK53465 and DK61594, and VA Merit Review, to BAM. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Molitoris, B., Melnikov, V., Okusa, M. et al. Technology Insight: biomarker development in acute kidney injury—what can we anticipate?. Nat Rev Nephrol 4, 154–165 (2008). https://doi.org/10.1038/ncpneph0723
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0723
This article is cited by
-
Plasma-specific microRNA response induced by acute exposure to aristolochic acid I in rats
Archives of Toxicology (2017)
-
Wnt4 is a novel biomarker for the early detection of kidney tubular injury after ischemia/reperfusion injury
Scientific Reports (2016)
-
The multifaceted role of the renal microvasculature during acute kidney injury
Pediatric Nephrology (2016)
-
Biomarkers for early diagnosis of AKI in the ICU: ready for prime time use at the bedside?
Annals of Intensive Care (2012)
-
A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals
Kidney International (2012)