Abstract
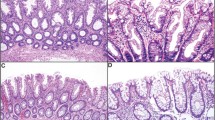
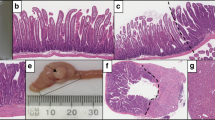
Serrated adenomas (SA) of the colorectum show features intermediate between hyperplastic polyps (HP) and adenomas. HP and SA are related lesions and there is now strong evidence for a 'serrated-polyp pathway' to colorectal cancer (CRC) that is largely independent of the classic adenoma-to-carcinoma sequence. A recently recognized lesion in this pathway is a HP variant characterized by relatively large size, atypical histology and proximal location in the colorectum. This HP variant has been given a variety of names in the literature including 'sessile SA' and 'type I SA'. Because this lesion lacks the traditional cytology of colorectal adenoma and in order to avoid confusion with SA, it is referred to in this review as sessile serrated polyp. SA are characterized by a heterogeneous group of changes at the molecular level, but a high proportion have BRAF mutations and DNA methylation. They may develop in HP or sessile serrated polyps, or may arise de novo. In the serrated-polyp pathway, the advent of genetic instability is likely to be an important rate-limiting step that drives rapid neoplastic evolution. Methylation and inactivation of the DNA repair genes MLH1 and MGMT (O-6-methylguanine-DNA methyltransferase) have been proposed as critical steps leading to genetic instability. Stretches of DNA rich in the bases guanine and cytosine (CpG islands; where p represents a phosphodiester bond linking adjacent cytosine and guanine bases) that are normally unmethylated may become methylated in malignant human colorectal tumors. Subsets of colorectal cancers with an unusually high number of methylated CpG islands have been described as having the 'CpG-island-methylator phenotype'. It is possible that many, if not all, CRCs with the CpG-island-methylator phenotype evolve through the serrated-polyp pathway that would, therefore, explain approximately 20% of all CRCs. The current lack of guidelines for managing serrated polyps may explain the static incidence of proximal CRC, despite the falling incidence rates for left-sided CRC during the same time period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hamilton SR and Aaltonen LA (2000) World Health Organization Classification of Tumours: Pathology and Genetics, Lyon: IARC Press
Kaye GI et al. (1973) Comparative electron microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. Variations in cellular structure relative to the process of epithelial differentiation. Gastroenterology 64: 926–945
Muto T et al. (1975). The evolution of cancer of the colon and rectum. Cancer 36: 2251–2270
Longacre TA and Fenoglio-Preiser CM (1990) Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 14: 524–537
Jass JR (2001) Serrated route to colorectal cancer: back street or super highway? J Pathol 193: 283–285
Jass JR et al. (1984) A morphologic and histochemical study of metaplastic polyps of the colorectum. Cancer 53: 510–515
Goldman H et al. (1970) Nature and significance of hyperplastic polyps of the human colon. Arch Pathol 89: 349–354
Urbanski SJ et al. (1984) Mixed hyperplastic adenomatous polyps—an underdiagnosed entity. Report of a case of adenocarcinoma arising within a mixed hyperplastic adenomatous polyp. Am J Surg Pathol 8: 551–556
Matsumoto T et al. (1999) Serrated adenoma of the colorectum: colonoscopic and histologic features. Gastrointest Endosc 49: 736–742
Miwa S et al. (2005) Clinicopathologic differences among subtypes of serrated adenomas of the colorectum. Hepatogastroenterology 52: 437–440
Rubio CA et al. (1996) Flat serrated adenomas of the colorectal mucosa in Japanese patients. In vivo 10: 339–344
Komori K et al. (2003) Proliferation kinetics and apoptosis of serrated adenoma of the colorectum. Pathol Int 53: 277–283
Kang M et al. (1997) Ki-67, p53, and Bcl-2 expression of serrated adenomas of the colon. Am J Surg Pathol 21: 417–423
Ban S (1999) Small hyperplastic polyps of the colorectum showing deranged cell organization: a lesion considered to be a serrated adenoma? Am J Surg Pathol 23: 1158–1160
Mitomi H et al. (2003) Different apoptotic activity and p21WAF1/CIP1, but not p27Kip1, expression in serrated adenomas as compared with traditional adenomas and hyperplastic polyps of the colorectum. J Cancer Res Clin Oncol 129: 449–455
Ladas SD et al. (2005) Cell turnover of serrated adenomas. J Pathol 206: 62–67
Iino H et al. (1999) DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 52: 5–9
Kudo T et al. (2004) Small invasive colonic cancer occurring in a hyperplastic polyp. Endoscopy 36: 825–828
Oh K et al. (2005) Support for hMLH1 and MGMT silencing as a mechanism of tumorigenesis in the hyperplastic-adenoma-carcinoma (serrated) carcinogenic pathway in the colon. Hum Pathol 36: 101–111
Otori K et al. (1997) High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut 40: 660–663
Ajioka Y et al. (1998) Infrequent K-ras codon 12 mutation in serrated adenomas of human colorectum. Gut 42: 680–684
Chan TL et al. (2003) BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res 63: 4878–4881
Jass JR et al. (2002) Emerging concepts in colorectal neoplasia. Gastroenterology 123: 862–876
Kambara T et al. (2004) BRAF mutation and CpG island methylation: an alternative pathway to colorectal cancer. Gut 53: 1137–1144
Tateyama H et al. (2002) Apoptosis index and apoptosis-related antigen expression in serrated adenoma of the colorectum: the saw-toothed structure may be related to inhibition of apoptosis. Am J Surg Pathol 26: 249–256
Jass JR (2004) Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol 2: 1–8
Torlakovic E et al. (2003) Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 27: 65–81
Biemer-Huttmann AE et al. (1999) Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem 47: 1039–1047
Yao T et al. (1999) 'Serrated' adenoma of the colorectum, with reference to its gastric differentiation and its malignant potential. J Pathol 187: 511–517
Cooper HS et al. (1979) Adenomatous and carcinomatous changes within hyperplastic colonic epithelium. Dis Colon Rectum 22: 152–156
Goldstein NS et al. (2003) Hyperplastic-like colon polyps that preceded microsatellite unstable adenocarcinomas. Am J Clin Pathol 119: 778–796
O'Brien MJ et al. (2004) Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol 28: 423–434
Torlakovic E and Snover DC (1996) Serrated adenomatous polyposis in humans. Gastroenterology 110: 748–755
Lazarus R et al. (2005) The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol 123: 349–359
Matsumoto T et al. (2002) Serrated adenoma in familial adenomatous polyposis: relation to germline APC gene mutation. Gut 50: 402–404
Bonte H and Flejou JF (2003) Patterns of expression of MMR proteins in serrated adenomas and other polyps of the colorectum. Gut 52: 611
Hiyama T et al. (1998) Frequent p53 gene mutations in serrated adenomas of the colorectum. J Pathol 186: 131–139
Fogt F et al. (2002) Genetic alterations in serrated adenomas: comparison to conventional adenomas and hyperplastic polyps. Hum Pathol 33: 87–91
Uchida H et al. (1998) Genetic alterations of mixed hyperplastic adenomatous polyps in the colon and rectum. Jpn J Cancer Res 89: 299–306
Sawyer EJ et al. (2002) Molecular characteristics of serrated adenomas. Gut 51: 200–206
Yamamoto T et al. (2003) No major tumorigenic role for beta-catenin in serrated as opposed to conventional colorectal adenomas. Br J Cancer 89: 152–157
Yang S et al. (2004) BRAF and KRAS mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol 28: 1452–1459
Park SJ et al. (2003) Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol 162: 815–822
Konishi K et al. (2004) Molecular differences between serrated and conventional colorectal adenomas. Clin Cancer Res 10: 3082–3090
Whitehall VL et al. (2001) Methylation of O-6-methylguanine-DNA methyltransferase characterizes a subset of colorectal cancer with low level DNA microsatellite instability. Cancer Res 61: 827–830
Hawkins NJ and Ward RL (2001) Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst 93: 1307–1313
Issa JP (2004) CpG island methylator phenotype in cancer. Nature Rev Cancer 4: 988–993
Toyota M et al. (1999) CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 96: 8681–8686
Hawkins N et al. (2002) CpG island methylation in sporadic colorectal cancer and its relationship to microsatellite instability. Gastroenterology 122: 1376–1387
Whitehall VL et al. (2002) Morphological and molecular heterogeneity within non-microsatellite instability-high colorectal cancer. Cancer Res 62: 6011–6014
Yamashita K et al. (2003) Genetics supersedes epigenetics in colon cancer phenotype. Cancer Cell 4: 121–131
Chirieac LR et al. (2005) Phenotype of microsatellite-stable colorectal carcinomas with CpG island methylation. Am J Surg Pathol 29: 429–436
Jass JR et al. (2000) Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut 47: 43–49
Frazier ML et al. (2003) Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res 63: 4805–4808
Young J et al. (2005) BRAF mutation and variable levels of microsatellite instability characterize a syndrome of familial colorectal cancer. Clin Gastroenterol Hepatol 3: 254–263
Beach R et al. (2005) BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol 166: 1069–1075
Cox AD and Der CJ (2003) The dark side of Ras: regulation of apoptosis. Oncogene 22: 8999–9006
Makinen MJ et al. (2001) Colorectal carcinoma associated with serrated adenoma—prevalence, histological features, and prognosis. J Pathol 193: 286–294
Higuchi T and Jass JR (2004) My approach to serrated polyps of the colorectum. J Clin Pathol 57: 682–686
Eide TJ (1986) Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer 38: 173–176
Yamauchi T et al. (2002) Serrated adenoma developing into advanced colon cancer in 2 years. J Gastroenterol 37: 467–470
Rabeneck L et al. (2003) Is there a true "shift" to the right colon in the incidence of colorectal cancer? Am J Gastroenterol 98: 1400–1409
Arao J et al. (2001) Cyclooxygenase-2 is overexpressed in serrated adenoma of the colorectum. Dis Colon Rectum 44: 1319–1323
Acknowledgements
This work was supported by the Canadian Institutes of Health Research, through funding of the project 'Serrated Polyps and Colorectal Cancer' (MOP-67206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- DYSPLASIA
-
A structural abnormality at the cell and tissue level that may be precancerous
- ANOIKIS
-
A type of apoptosis, of cells that have lost contact with the extracellular matrix or that interact with the matrix through an inappropriate integrin–matrix combination
- GOBLET CELLS
-
Specialized mucus-secreting epithelial cells that are found in the mucous membranes of the intestinal and respiratory tracts
- MUTL HOMOLOG 1 (MLH1)
-
A DNA mismatch repair gene found on chromosome 3p that accounts for a large proportion of hereditary nonpolyposis colorectal cancers
- BIMODAL DISTRIBUTION
-
A statistical term that describes the distribution of two distinct sets of values that measurements tend to center around
- ANEUPLOIDY
-
Cell population that has additions or deletions of whole chromosomes from the expected balanced diploid number of chromosomes
- RAS ASSOCIATION (RALGDS/AF-6) DOMAIN FAMILY 1 (RASSF1)
-
Encodes a protein similar to the Ras effector proteins; loss or altered expression of this gene is associated with the pathogenesis of a variety of cancers and correlates with the hypermethylation of its CpG-island promoter region
Rights and permissions
About this article
Cite this article
Jass, J. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Rev Clin Oncol 2, 398–405 (2005). https://doi.org/10.1038/ncponc0248
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0248
This article is cited by
-
Serrated colonic polyps: dispelling myths
Abdominal Radiology (2016)
-
A deleterious RNF43 germline mutation in a severely affected serrated polyposis kindred
Human Genome Variation (2015)
-
Aberrant expression of annexin A10 is closely related to gastric phenotype in serrated pathway to colorectal carcinoma
Modern Pathology (2015)
-
Genome-wide linkage analysis and tumoral characterization reveal heterogeneity in familial colorectal cancer type X
Journal of Gastroenterology (2015)
-
Impact of Chromoscopy on Adenoma Detection in Patients With Lynch Syndrome: A Prospective, Multicenter, Blinded, Tandem Colonoscopy Study
American Journal of Gastroenterology (2015)