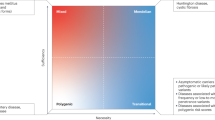
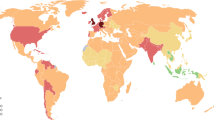
Abstract
Most rheumatic diseases are complex disorders for which pathogenetic mechanisms are poorly understood. Nonetheless, increasing evidence suggests that many of these illnesses result from one or more specific environmental exposures in genetically susceptible individuals. Although much progress has been made over the past few decades in advancing our knowledge of the genetics of rheumatic diseases, few studies have assessed environmental features and understanding of which exposures are important in pathogenesis remains limited. In this article, we review the difficulties inherent in deciphering the interacting environmental and genetic risk factors for rheumatic diseases, the current state of knowledge of infectious and noninfectious risk factors, possible mechanisms by which environmental exposures might induce pathologic processes and future directions. The advances in technologies and statistical approaches, development of collaborating consortia and focused resources that have resulted in the explosion of genetic information must now be applied to environmental studies so we can eventually interrupt pathogenesis before the onset of disease and transform the practice of medicine from curative to pre-emptive paradigms.
Key Points
-
Complementary lines of evidence point to the role of environmental factors in the pathogenesis of rheumatic diseases
-
In addition to well-described cases of drug-induced disease, epidemiologic data support a role for inhaled silica, solvents, pesticides, tobacco smoke and DNA viruses in triggering many rheumatic diseases
-
Mechanisms are ill-defined for all environmentally-associated rheumatic diseases
-
Integrated collaborative approaches, focused resources and better tools, including validated exposure biomarkers and questionnaires, are needed to define additional environmental risk factors and, ultimately, prevent the development of certain forms of rheumatic disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Miller FW et al. (2000) Approaches for identifying and defining environmentally associated rheumatic disorders. Arthritis Rheum 43: 243–249
Rodriguez-Reyna TS and Alarcon-Segovia D (2006) The different faces of shared autoimmunity. Autoimmun Rev 5: 86–88
Mackay IR (2005) The etiopathogenesis of autoimmunity. Semin Liver Dis 25: 239–250
van der Helm-van Mil AH et al. (2005) Understanding the genetic contribution to rheumatoid arthritis. Curr Opin Rheumatol 17: 299–304
Sarzi-Puttini P et al. (2005) Drug-induced lupus erythematosus. Autoimmunity 38: 507–518
Bramwell B (1914) Diffuse scleroderma: its frequency and occurrence in stonemasons; its treatment by fibrinolysin: elevations of temperature due to fibrinolysin injections. Edinburg Med J 12: 387
Calvert GM et al. (2003) Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 60: 122–129
Stolt P et al. (2003) Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis 62: 835–841
Parks CG et al. (2002) Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: A population-based, case-control study in the Southeastern United States. Arthritis Rheum 46: 1840–1850
Diot E et al. (2002) Systemic sclerosis and occupational risk factors: a case-control study. Occup Environ Med 59: 545–549
Bovenzi M et al. (2004) A case-control study of occupational exposures and systemic sclerosis. Int Arch Occup Environ Health 77: 10–16
Nuyts GD et al. (1995) Wegener granulomatosis is associated to exposure to silicon compounds: a case-control study. Nephrol Dial Transplant 10: 1162–1165
Hogan SL et al. (2001) Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol 12: 134–142
Gregorini G et al. (1993) Association between silica exposure and necrotizing crescentic glomerulonephritis with p-ANCA and anti-MPO antibodies: a hospital-based case-control study. Adv Exp Med Biol 336: 435–440
Lane SE et al. (2003) Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheum 48: 814–823
De Roos AJ et al. (2005) Rheumatoid arthritis among women in the agricultural health study: risk associated with farming activities and exposures. Ann Epidemiol 15: 762–770
Nietert PJ and Silver RM (2000) Systemic sclerosis: environmental and occupational risk factors. Curr Opin Rheumatol 12: 520–526
Sverdrup B et al. (2005) Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res Ther 7: R1296–R1303
Cooper GS et al. (2004) Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol 31: 1928–1933
Han SH (2004) Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis 8: 403–418
Anzilotti C et al. (2006) Antibodies to Viral Citrullinated Peptide in Rheumatoid Arthritis. J Rheumatol 33: 647–651
Parks CG et al. (2005) Association of Epstein-Barr virus with systemic lupus erythematosus: Effect modification by race, age, and cytotoxic T lymphocyte-associated antigen 4 genotype. Arthritis Rheum 52: 1148–1159
Yu SF et al. (2005) Detecting Epstein-Barr virus DNA from peripheral blood mononuclear cells in adult patients with systemic lupus erythematosus in Taiwan. Med Microbiol Immunol (Berlin) 194: 115–120
Rider JR et al. (1997) Human cytomegalovirus infection and systemic lupus erythematosus. Clin Exp Rheumatol 15: 405–409
Pope JE et al. (2004) Close association of herpes zoster reactivation and systemic lupus erythematosus (SLE) diagnosis: case-control study of patients with SLE or noninflammatory nusculoskeletal disorders. J Rheumatol 31: 274–279
Cooper GS et al. (2002) Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. J Clin Epidemiol 55: 982–989
Strom BL et al. (1994) Shingles, allergies, family medical history, oral contraceptives, and other potential risk factors for systemic lupus erythematosus. Am J Epidemiol 140: 632–642
James JA et al. (2001) Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum 44: 1122–1126
James JA et al. (1997) An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest 100: 3019–3026
Caliskan R et al. (2005) The relationship between arthritis and human parvovirus B19 infection. Rheumatol Int 26: 7–11
Hajeer AH et al. (1994) Influence of previous exposure to human parvovirus B19 infection in explaining susceptibility to rheumatoid arthritis: an analysis of disease discordant twin pairs. Ann Rheum Dis 53: 137–139
Oguz F et al. (2002) Parvovirus B19 in the acute arthropathies and juvenile rheumatoid arthritis. J Paediatr Child Health 38: 358–362
Mamyrova G et al. (2005) Parvovirus B19 and onset of juvenile dermatomyositis. JAMA 294: 2170–2171
Lyon MG et al. (1989) Predisposing factors in polymyositis-dermatomyositis: results of a nationwide survey. J Rheumatol 16: 1218–1224
Koch MJ et al. (1976) Childhood polymyositis: a case-control study. Am J Epidemiol 104: 627–631
Grimes DA et al. (1985) Systemic lupus erythematosus and reproductive function: a case-control study. Am J Obstet Gynecol 153: 179–186
Minami Y et al. (1993) Female systemic lupus erythematosus in Miyagi Prefecture, Japan: a case-control study of dietary and reproductive factors. Tohoku J Exp Med 169: 245–252
Nagata C et al. (1995) Systemic lupus erythematosus: a case-control epidemiologic study in Japan. Int J Dermatol 34: 333–337
Cooper GS et al. (2002) Hormonal and reproductive risk factors for development of systemic lupus erythematosus: results of a population-based, case-control study. Arthritis Rheum 46: 1830–1839
Sanchez-Guerrero J et al. (1997) Past use of oral contraceptives and the risk of developing systemic lupus erythematosus. Arthritis Rheum 40: 804–808
Bengtsson AA et al. (2002) Risk factors for developing systemic lupus erythematosus: a case-control study in southern Sweden. Rheumatology (Oxford) 41: 563–571
Okada S et al. (2003) Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum 48: 2285–2293
Howson CP et al. (1991) Adverse Effects of Pertussis and Rubella Vaccines: A Report of the Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines. Washington, DC: National Academies Press
Cooper GS and Parks CG (2004) Occupational and environmental exposures as risk factors for systemic lupus erythematosus. Curr Rheumatol Rep 6: 367–374
Klareskog L et al. (2006) A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 54: 38–46
Padyukov L et al. (2004) A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 50: 3085–3092
Pattison DJ et al. (2004) Dietary risk factors for the development of inflammatory polyarthritis: evidence for a role of high level of red meat consumption. Arthritis Rheum 50: 3804–3812
Merlino LA et al. (2004) Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 50: 72–77
Herrmann M et al. (2000) Stress and rheumatic diseases. Rheum Dis Clin North Am 26: 737–763, viii
Sarkar K and Miller FW (2004) Possible roles and determinants of microchimerism in autoimmune and other disorders. Autoimmun Rev 3: 454–463
Leff RL et al. (1988) Epidemiology of adult idiopathic inflammatory myopathy: A distinct clinical onset in patients with anti-Jo-1 antibodies. Arthritis Rheum 31: D113–S121
Kuhn A and Beissert S (2005) Photosensitivity in lupus erythematosus. Autoimmunity 38: 519–529
Furukawa F et al. (1990) Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J Invest Dermatol 94: 77–85
Ueki A et al. (1994) Polyclonal human T-cell activation by silicate in vitro. Immunology 82: 332–335
Koeger AC et al. (1995) Silica-associated connective tissue disease. A study of 24 cases. Medicine (Baltimore) 74: 221–237
Guilherme L et al. (2005) Molecular pathogenesis of rheumatic fever and rheumatic heart disease. Expert Rev Mol Med 7: 1–15
McClain MT et al. (2005) Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med 11: 85–89
Duna GF et al. (1998) Wegener's granulomatosis: role of environmental exposures. Clin Exp Rheumatol 16: 669–674
Acknowledgements
We thank Drs Lori Love, Glinda Cooper, John Harley and Lisa Rider for many useful discussions and concepts in this area and we are grateful to Bradley Otterson and David Green for their technical assistance. This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences at the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Gourley, M., Miller, F. Mechanisms of Disease: environmental factors in the pathogenesis of rheumatic disease. Nat Rev Rheumatol 3, 172–180 (2007). https://doi.org/10.1038/ncprheum0435
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0435
This article is cited by
-
Slicing and dicing myositis for cures and prevention
Nature Reviews Rheumatology (2021)
-
Lifestyle and behavioral modifications made by patients with interstitial cystitis
Scientific Reports (2021)
-
Inflammasomes and Childhood Autoimmune Diseases: A Review of Current Knowledge
Clinical Reviews in Allergy & Immunology (2021)
-
Risk factors and disease mechanisms in myositis
Nature Reviews Rheumatology (2018)
-
Environmental Basis of Autoimmunity
Clinical Reviews in Allergy & Immunology (2016)