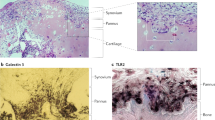
Abstract
Synovial tissue lines the noncartilaginous surfaces of synovial joints and supplies these avascular structures with nutrients. In diseases such as rheumatoid arthritis, inflammation of the synovial tissue—synovitis—induces diffuse damage to the joints. The presence of functional receptors for glucocorticoids, androgens and estrogens in synoviocytes might link inflammation and the endocrine system at the local level. Synovial tissue could be regarded as an intracrine tissue, whereby active steroids influence the cells in which they are synthesized, without their release into the extracellular space. An increase in the peripheral metabolism of sex steroids is characteristic of rheumatoid synovitis, with an augmented ratio of estrogen to androgen occurring in both male and female patients. Changes in the peripheral nervous system at the site of local inflammation are also hallmarks of synovitis in rheumatoid arthritis. In the chronic phase of synovitis, sympathetic nerve fibers are lost; by contrast, sensory nerve fibers sprout into the inflamed tissue. Complex interactions occur between the endocrine, nervous and immune systems during synovitis. In particular, studying neuroendocrine–immune interactions in the inflamed synovium will potentially uncover new mechanisms in the pathophysiology of rheumatoid arthritis and might lead to new methods of therapeutic intervention.
Key Points
-
The presence of functional glucocorticoid, androgen and estrogen receptors in synoviocytes links the endocrine system and the immune response/inflammation at a local level
-
The synovial tissue might be considered as an intracrine tissue, whereby the effects of active steroids occur in the same cells in which they are made
-
Increased peripheral metabolism of sex steroids occurs in rheumatoid synovitis, with an augmented estrogen to androgen ratio in both sexes
-
Changes in the peripheral nervous system at the site of local inflammation are also hallmarks of synovitis in rheumatoid arthritis
-
Complex and changing interactions between the endocrine, nervous and immune systems occur during synovitis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tak PP (2006) Examination of the synovium and synovial fluid. In Rheumatoid arthritis, 229–241 (Eds Firestein GS et al.) Oxford: Oxford University Press
Straub RH and Cutolo M (2001) Involvement of the hypothalamic–pituitary–adrenal/gonadal axis and the peripheral nervous system in rheumatoid arthritis: viewpoint based on a systemic pathogenetic role. Arthritis Rheum 44: 493–507
Hench PS et al. (1950) Effect of cortisone and pituitary adrenocorticotropic hormone (ACTH) on rheumatic diseases. J Am Med Assoc 40: 1327–1335
Bland JH and Eddy WM (1968) Hemiplegia and rheumatoid hemiarthritis. Arthritis Rheum 11: 72–80
Levine JD et al. (1984) Intraneuronal substance P contributes to the severity of experimental arthritis. Science 226: 547–549
Besedovsky HO and Del Rey A (1996) Immune-neuro-endocrine interactions. Endocr Rev 17: 64–102
Ader R (2007) Psychoneuroimmunology. San Diego: Academic Press
Bijlsma JW et al. (2002) Neuroendocrine immune mechanisms in rheumatic diseases. Trends Immunol 23: 59–61
Schmidt M et al. (2005) Androgen conversion in osteoarthritis and rheumatoid arthritis synoviocytes—androstenedione and testosterone inhibit estrogen formation and favor production of more potent 5alpha-reduced androgens. Arthritis Res Ther 7: R938–R948
Miller LE et al. (2000) The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J 14: 2097–2107
Labrie F (1991) Intracrinology. Mol Cell Endocrinol 78: 113–118
Labrie F et al. (2000) Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol 25: 1–16
Martel C et al. (1992) Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues. J Steroid Biochem Mol Biol 41: 597–603
Cutolo M et al. (1992) Evidence for the presence of androgen receptors in the synovial tissue of rheumatoid arthritis patients and healthy controls. Arthritis Rheum 35: 1007–1015
Cutolo M et al. (1993) Presence of estrogen-binding sites on macrophage-like synoviocytes and CD8+, CD29+, CD45RO+ T lymphocytes in normal and rheumatoid synovium. Arthritis Rheum 36: 1087–1097
Cutolo M et al. (1998) Androgen and estrogen receptors are present in primary cultures of human synovial macrophages. J Clin Endocrinol Metab 81: 820–827
Simard J and Gingras S (2001) Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol Cell Endocrinol 171: 25–40
Honma S et al. (2002) The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr J 49: 371–377
Le Bail J et al. (2001) Aromatase in synovial cells from postmenopausal women. Steroids 66: 749–757
Macdiarmid F et al. (1994) Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor alpha. Mol Cell Endocrinol 106: 17–21
Castagnetta LA et al. (2003) Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol 30: 2597–2605
Cutolo M et al. (2004) Synovial fluid estrogens in rheumatoid arthritis. Autoimmun Rev 3: 193–198
Cutolo M et al. (2003) New roles for estrogens in rheumatoid arthritis. Clin Exp Rheumatol 21: 687–690
Capellino S et al. (2007) Quantitative determination of steroid hormone receptor positive cells in the synovium of patients with rheumatoid arthritis and osteoarthritis: is there a link to inflammation? Ann Rheum Dis 66: 53–58
Eijsbouts AM et al. (2005) Hypothalamic-pituitary-adrenal axis activity in patients with rheumatoid arthritis. Clin Exp Rheumatol 23: 658–664
Schmidt M et al. (2005) Reduced capacity for the reactivation of glucocorticoids in rheumatoid arthritis synovial cells: possible role of the sympathetic nervous system? Arthritis Rheum 52: 1711–1720
Harbuz MS et al. (2002) Hypothalamo-pituitary-adrenal axis and chronic immune activation. Ann N Y Acad Sci 992: 99–106
van Rossum EF and Lamberts SW (2006) Glucocorticoid resistance syndrome: A diagnostic and therapeutic approach. Best Pract Res Clin Endocrinol Metab 20: 6–26
Cutolo M et al. (2006) Serum cytokines and steroidal hormones in polymyalgia rheumatica and elderly-onset rheumatoid arthritis. Ann Rheum Dis 65: 1438–1443
Straub RH et al. (1998) Association of humoral markers of inflammation and dehydroepiandrosterone sulfate or cortisol serum levels in patients with chronic inflammatory bowel disease. Am J Gastroenterol 98: 2197–2202
Maestroni GJ et al. (2002) Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann N Y Acad Sci 966: 271–275
Maestroni GJ et al. (2002) Melatonin in rheumatoid arthritis: a disease-promoting and modulating hormone? Clin Exp Rheumatol 20: 872–873
Cutolo M et al. (2005) Nocturnal hormones and clinical rhythms in rheumatoid arthritis. Ann N Y Acad Sci 1051: 372–381
Doria A et al. (2006) Th2 immune deviation induced by pregnancy: the two faces of autoimmune rheumatic diseases. Reprod Toxicol 22: 234–241
Druckmann R and Druckmann MA (2005) Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol 97: 389–396
Szekeres-Bartho J et al. (2001) Progesterone as an immunomodulatory molecule. Int Immunopharmacol 1: 1037–1048
Rovensky J et al. (2005) Hormone concentrations in synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol 23: 292–296
Härle P et al. (2005) An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum 2: 1305–1313
Levine JD et al. (1998) Beta 2-adrenergic mechanisms in experimental arthritis. Proc Natl Acad Sci USA 85: 4553–4556
Dhabhar FS and McEwen BS (2001) Bidirectional effects of stress and glucocorticoid hormones on immune function: possible explanations for paradoxical observations. In Psychoneuroimmunology, 301–338 (Eds Ader R et al.) San Diego: Academic Press
Straub RH et al. (2000) Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol 67: 553–558
Kohm AP and Sanders VM (2000) Norepinephrine: a messenger from the brain to the immune system. Immunology Today 21: 539–542
Elenkov IJ et al. (2000) The sympathetic nervous system—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638
Miller LE et al. (2004) Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum 50: 1156–1163
Baerwald CG et al. (1997) Impaired sympathetic influence on the immune response in patients with rheumatoid arthritis due to lymphocyte subset-specific modulation of beta 2-adrenergic receptors. Br J Rheumatol 36: 1262–1269
Heijnen CJ et al. (1996) Functional alpha 1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol 71: 223–226
Miller LE et al. (2002) Norepinephrine from synovial tyrosine hydroxylase positive cells is a strong indicator of synovial inflammation in rheumatoid arthritis. J Rheumatol 29: 427–435
Bijlsma JW et al. (2006) Neuroendocrine immune system involvement in rheumatology. Ann N Y Acad Sci 1069: xviii–xxiv
Bijlsma JWJ et al. (2005) Clinical aspects of immune neuroendocrine mechanisms in rheumatic diseases. Rheum Dis Clin North Am 31: xiii–xvi
Forslind K et al.; the BARFOT Study Group (2007) Sex: a major predictor of remission in early rheumatoid arthritis? Ann Rheum Dis 66: 46–52
Kvien TK et al. (2006) Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci 1069: 212–222
Cutolo M et al. (2006) Anti-TNF and sex hormones. Ann N Y Acad Sci 1069: 391–400
Straub RH et al. (2006) Tumor necrosis factor-neutralizing therapies improve altered hormone axes: an alternative mode of antiinflammatory action. Arthritis Rheum 54: 2039–2046
Tengstrand B et al. (2003) Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. J Rheumatol 30: 2338–2343
Straub RH et al. (2005) Sex hormone concentrations in patients with rheumatoid arthritis are not normalized during 12 weeks of anti-tumor necrosis factor therapy. J Rheumatol 32: 1253–1258
Komi J et al. (2001) Non-steroidal anti-oestrogens inhibit the differentiation of synovial macrophages into dendritic cells. Rheumatology (Oxford) 40: 185–191
Cutolo M et al. (1997) Testosterone metabolism and cyclosporin A treatment in rheumatoid arthritis. Br J Rheumatol 36: 433–439
Taneja V et al. (2007) New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum 56: 69–78
Straub RH et al. (2002) Anti-inflammatory cooperativity of corticosteroids and norepinephrine in rheumatoid arthritis synovial tissue in vivo and in vitro. FASEB J 16: 993–1005
Sternberg EM (2006) Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol 6: 318–328
Cutolo M and Straub RH (2006) Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation 13: 277–282
Straub RH and Cutolo M (2007) Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum 56: 399–408
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cutolo, M., Straub, R. & Bijlsma, J. Neuroendocrine–immune interactions in synovitis. Nat Rev Rheumatol 3, 627–634 (2007). https://doi.org/10.1038/ncprheum0601
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0601
This article is cited by
-
Perspectives of colon-specific drug delivery in the management of morning symptoms of rheumatoid arthritis
Inflammopharmacology (2023)
-
Adolescent-Onset Depression: Are Obesity and Inflammation Developmental Mechanisms or Outcomes?
Child Psychiatry & Human Development (2015)
-
Circadian rhythms in rheumatology - a glucocorticoid perspective
Arthritis Research & Therapy (2014)
-
Urokinase Plasminogen Activator System in Synovial Fibroblasts from Osteoarthritis Patients: Modulation by Inflammatory Mediators and Neuropeptides
Journal of Molecular Neuroscience (2014)
-
Neuroendokrin-immune Interaktionen bei rheumatischen Krankheiten
Zeitschrift für Rheumatologie (2010)