Abstract
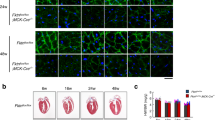
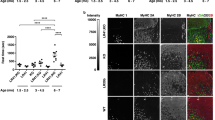
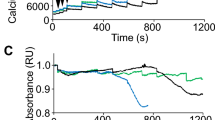
Myotonic muscular dystrophy (DM1) is the most common inherited neuromuscular disorder in adults and is considered the first example of a disease caused by RNA toxicity. Using a reversible transgenic mouse model of RNA toxicity in DM1, we provide evidence that DM1 is associated with induced NKX2-5 expression. Transgene expression resulted in cardiac conduction defects, increased expression of the cardiac-specific transcription factor NKX2-5 and profound disturbances in connexin 40 and connexin 43. Notably, overexpression of the DMPK 3′ UTR mRNA in mouse skeletal muscle also induced transcriptional activation of Nkx2-5 and its targets. In human muscles, these changes were specific to DM1 and were not present in other muscular dystrophies. The effects on NKX2-5 and its downstream targets were reversed by silencing toxic RNA expression. Furthermore, using Nkx2-5+/− mice, we show that NKX2-5 is the first genetic modifier of DM1-associated RNA toxicity in the heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Harper, P.S. Myotonic Dystrophy (W.B. Saunders, London, 1989).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Pelargonio, G., Dello Russo, A., Sanna, T., De Martino, G. & Bellocci, F. Myotonic dystrophy and the heart. Heart 88, 665–670 (2002).
Tapscott, S.J. & Thornton, C.A. Biomedicine. Reconstructing myotonic dystrophy. Science 293, 816–817 (2001).
Mankodi, A. et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773 (2000).
Amack, J.D., Paguio, A.P. & Mahadevan, M.S. Cis and trans effects of the myotonic dystrophy (DM) mutation in a cell culture model. Hum. Mol. Genet. 8, 1975–1984 (1999).
Savkur, R.S., Philips, A.V. & Cooper, T.A. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 29, 40–47 (2001).
Charlet, B.N. et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell 10, 45–53 (2002).
Mankodi, A. et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 10, 35–44 (2002).
Taneja, K.L., McCurrach, M., Schalling, M., Housman, D. & Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 128, 995–1002 (1995).
Liquori, C.L. et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867 (2001).
Ranum, L.P. & Day, J.W. Myotonic dystrophy: RNA pathogenesis comes into focus. Am. J. Hum. Genet. 74, 793–804 (2004).
Berul, C.I. et al. DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J. Clin. Invest. 103, R1–R7 (1999).
Berul, C.I., Maguire, C.T., Gehrmann, J. & Reddy, S. Progressive atrioventricular conduction block in a mouse myotonic dystrophy model. J. Interv. Card. Electrophysiol. 4, 351–358 (2000).
Mankodi, A., Lin, X., Blaxall, B.C., Swanson, M.S. & Thornton, C.A. Nuclear RNA foci in the heart in myotonic dystrophy. Circ. Res. 97, 1152–1155 (2005).
Mahadevan, M.S. et al. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat. Genet. 38, 1066–1070 (2006).
Gaussin, V. Offbeat mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 280, 1022–1026 (2004).
Lo, C.W. Role of gap junctions in cardiac conduction and development: insights from the connexin knockout mice. Circ. Res. 87, 346–348 (2000).
Akazawa, H. & Komuro, I. Cardiac transcription factor Csx/Nkx2–5: Its role in cardiac development and diseases. Pharmacol. Ther. 107, 252–268 (2005).
Schott, J.J. et al. Congenital heart disease caused by mutations in the transcription factor NKX2–5. Science 281, 108–111 (1998).
Biben, C. et al. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2–5. Circ. Res. 87, 888–895 (2000).
Tanaka, M. et al. A mouse model of congenital heart disease: cardiac arrhythmias and atrial septal defect caused by haploinsufficiency of the cardiac transcription factor Csx/Nkx2.5. Cold Spring Harb. Symp. Quant. Biol. 67, 317–325 (2002).
Kasahara, H. et al. Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein. J. Clin. Invest. 108, 189–201 (2001).
Wakimoto, H., Kasahara, H., Maguire, C.T., Izumo, S. & Berul, C.I. Developmentally modulated cardiac conduction failure in transgenic mice with fetal or postnatal overexpression of DNA nonbinding mutant Nkx2.5. J. Cardiovasc. Electrophysiol. 13, 682–688 (2002).
Lints, T.J., Parsons, L.M., Hartley, L., Lyons, I. & Harvey, R.P. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 119, 419–431 (1993).
Kasahara, H., Bartunkova, S., Schinke, M., Tanaka, M. & Izumo, S. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ. Res. 82, 936–946 (1998).
Riazi, A.M., Lee, H., Hsu, C. & Van Arsdell, G. CSX/Nkx2.5 modulates differentiation of skeletal myoblasts and promotes differentiation into neuronal cells in vitro. J. Biol. Chem. 280, 10716–10720 (2005).
Furling, D., Lemieux, D., Taneja, K. & Puymirat, J. Decreased levels of myotonic dystrophy protein kinase (DMPK) and delayed differentiation in human myotonic dystrophy myoblasts. Neuromuscul. Disord. 11, 728–735 (2001).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997).
Chen, C.P. et al. Molecular cytogenetic analysis of de novo dup(5)(q35.2q35.3) and review of the literature of pure partial trisomy 5q. Am. J. Med. Genet. A. 140, 1594–1600 (2006).
Kasahara, H. et al. Nkx2.5 homeoprotein regulates expression of gap junction protein connexin 43 and sarcomere organization in postnatal cardiomyocytes. J. Mol. Cell. Cardiol. 35, 243–256 (2003).
Bruneau, B.G. et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106, 709–721 (2001).
Linhares, V.L. et al. Transcriptional regulation of the murine Connexin40 promoter by cardiac factors Nkx2–5, GATA4 and Tbx5. Cardiovasc. Res. 64, 402–411 (2004).
Jay, P.Y. et al. Nkx2–5 mutation causes anatomic hypoplasia of the cardiac conduction system. J. Clin. Invest. 113, 1130–1137 (2004).
Pashmforoush, M. et al. Nkx2–5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 117, 373–386 (2004).
Wakimoto, H. et al. Cardiac electrophysiological phenotypes in postnatal expression of Nkx2.5 transgenic mice. Genesis 37, 144–150 (2003).
Teunissen, B.E. et al. Analysis of the rat connexin 43 proximal promoter in neonatal cardiomyocytes. Gene 322, 123–136 (2003).
Gorbe, A. et al. Transient upregulation of connexin43 gap junctions and synchronized cell cycle control precede myoblast fusion in regenerating skeletal muscle in vivo. Histochem. Cell Biol. 123, 573–583 (2005).
Araya, R. et al. Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J. Cell Sci. 118, 27–37 (2005).
Amack, J.D., Reagan, S.R. & Mahadevan, M.S. Mutant DMPK 3′-UTR transcripts disrupt C2C12 myogenic differentiation by compromising MyoD. J. Cell Biol. 159, 419–429 (2002).
Timchenko, N.A., Iakova, P., Cai, Z.J., Smith, J.R. & Timchenko, L.T. Molecular basis for impaired muscle differentiation in myotonic dystrophy. Mol. Cell. Biol. 21, 6927–6938 (2001).
Brown, C.O., III et al. The cardiac determination factor, Nkx2–5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J. Biol. Chem. 279, 10659–10669 (2004).
Liberatore, C.M., Searcy-Schrick, R.D., Vincent, E.B. & Yutzey, K.E. Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev. Biol. 244, 243–256 (2002).
Lien, C.L. et al. Control of early cardiac-specific transcription of Nkx2–5 by a GATA-dependent enhancer. Development 126, 75–84 (1999).
Prall, O.W. et al. An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128, 947–959 (2007).
Schwartz, R.J. & Olson, E.N. Building the heart piece by piece: modularity of cis-elements regulating Nkx2–5 transcription. Development 126, 4187–4192 (1999).
Elliott, D.A. et al. A tyrosine-rich domain within homeodomain transcription factor Nkx2–5 is an essential element in the early cardiac transcriptional regulatory machinery. Development 133, 1311–1322 (2006).
Mankodi, A. et al. Ribonuclear inclusions in skeletal muscle in myotonic dystrophy types 1 and 2. Ann. Neurol. 54, 760–768 (2003).
Langlois, M.A., Lee, N.S., Rossi, J.J. & Puymirat, J. Hammerhead ribozyme-mediated destruction of nuclear foci in myotonic dystrophy myoblasts. Mol. Ther. 7, 670–680 (2003).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408 (2001).
Acknowledgements
We thank P. Mahadevan for critically reading the manuscript. Human tissues were purchased from the University of Miami Brain and Tissue Bank. Transgenic mice were generated by the University of Wisconsin-Madison Transgenic Core Facility. All studies were done under the auspices of the University of Virginia Animal Care and Use Committee and Institutional Review Board. This work was supported by the Muscular Dystrophy Association (grant 3889) and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR052771).
Author information
Authors and Affiliations
Contributions
M.S.M. discovered NKX2-5 induction and performed mouse phenotypic analyses, immunohistochemistry and protein blotting. R.S.Y. performed molecular analyses and transfection assays. Q.Y. managed the mouse colony and performed mouse phenotypic analyses and tissue collection. C.D.F.-M. performed RT-PCR assays and analyses, and V.S. performed mouse experimental work and tissue staining. J.P. and C.A.T. provided human tissues. A.L.T. provided ECG machines and assistance in performing and analyzing ECGs. O.W.P. and R.P.H. provided Nkx2-5LacZ/+ mice and gave experimental advice, critical insight and comments. M.S.M. was responsible for conceptual design and execution.
Corresponding author
Ethics declarations
Competing interests
We have filed a patent application based on the results described in this manuscript
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Methods (PDF 233 kb)
Rights and permissions
About this article
Cite this article
Yadava, R., Frenzel-McCardell, C., Yu, Q. et al. RNA toxicity in myotonic muscular dystrophy induces NKX2-5 expression. Nat Genet 40, 61–68 (2008). https://doi.org/10.1038/ng.2007.28
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2007.28