Abstract
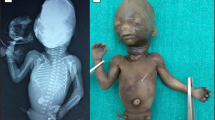
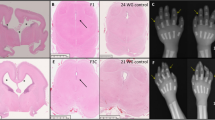
Sacral agenesis is a rare disorder of uncertain incidence1 that has been reported in diverse populations. Although usually sporadic and most commonly associated with maternal diabetes, there is a hereditary form which may occur in isolation or with a presacral mass (anterior meningocele and/or presacral teratoma) and anorectal abnormalities, which constitute the Currarino triad2 (MIM 176450). The radiological hallmark of hereditary sacral agenesis is a hemi-sacrum (sickle-shaped sacrum) with intact first sacral vertebra. Bowel obstruction is the usual neonatal presentation, but, unlike other neural tube defects, adult presentation is not uncommon. The major pathology is confined to the pelvic cavity and may present as a space-occupying lesion or meningitis due to ascending infection. All recurrences in families have been compatible with autosomal dominant inheritance except for those associated with the isomerism gene at Xq24–q27.1 (ref. 3). Several associated cytogenetic defects have been reported, including 7q deletions4. Previous studies failed to detect linkage to HLA markers5,6, but we now present evidence for a location on 7q36. The same region also contains a gene for holoprosencephaly, an early malformation of the extreme rostral end of the neural tube7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pang, D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery 32, 755–779 (1993).
Currarino, G., Coln, D. & Votteler, T. Triad of anorectal, sacral, and presacral anomalies. Am. J. Roentgenol. 137, 395–398 (1981).
Casey, B., Devoto, M., Jones, K.L. & Ballabio, A. Mapping a gene for familial situs abnormalities to human chromosome Xq24–q2701. Nature Genet. 5, 403–407 (1993).
Human Cytogenetics Database, version 1.0. (Oxford University Press Electronic Publishing, 1994).
Fellous, M. et al. A five-generation family with sacral agenesis and spina bifida: possible similarities with the mouse t-locus. Am. J. med Genet. 12, 465–487 (1982).
Chatkupt, S. et al. Linkage analysis of a candidate locus (HLA) in autosomal dominant sacral defect with anterior meningocele. Am. J. med. Genet. 52, 1–4 (1994).
Muenke, M. Holoprosencephaly as a genetic model for normal craniofacial development. Semin. dev. Biol. 5, 293–301 (1994).
O'Riordain, D.S., O'Connell, P.R. & Kirwan, W.O. Hereditary sacral agenesis with presacral mass and anorectal stenosis: the Currarino triad. Br. J. Surg. 78, 536–538 (1991).
Nour, S., Kumar, D. & Dickson, J. Anorectal malformations with sacral bony abnormalities. Arch. Dis. Child. 64, 1618–1620 (1989).
Green, E.D. et al. Integration of physical, genetic and cytogenetic maps of human chromosome 7: isolation and analysis of yeast artificial chromosome clones for 117 mapped genetic markers. Hum. molec. Genet. 3, 489–501 (1994).
Muller, F. & O'Rahilly, R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat Embryol. (Berlin) 176, 413–430 (1987).
Copp, A.J. & Brook, F.A. Does lumbosacral spina bifida arise by failure of neural folding or by defective canalisation? J. med. Genet. 26, 160–166 (1989).
Fan, C.M. & Tessier-Lavigne, M. Patterning of mammalian somites by surface ectroderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell 79, 1175–1186 (1994).
Basler, K., Edlund, T., Jessell, T.M. & Yamada, T. Control of cell pattern in the neural tube: regulation of cell differentiation by dirsalin-1, a novel TGB family member. Cell 73, 687–702 (1993).
Tam, P.P.L. The histogenetic capacity of tissues in the caudal end of the embryonic axis of the mouse. J. Embryol. exp. Morph. 82, 253–266 (1984).
Alles, A.J. & Sulik, K.K. Retinoic acid-induced spina bifida: evidence for a pathogenetic mechanism. Development 108, 73–81 (1990).
Lohnes, D. et al. Function of retinoic acid receptor gamma in the mouse. Cell 73, 643–658 (1993).
Chesley, P. Development of the short-tailed mutant in the house mouse. J. exp. Zool. 70, 429–459 (1935).
Koseki, H. et al. A role for Pax-1 as a mediator of notochordal signals during the dorsoventral specification of vertebrae. Development 119, 649–660 (1993).
Cohen, M.M.J. & Sulik, K.K. Perspectives on holoprosencephaly. Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J. Craniofacial Genet. Dev. Biol. 12, 196–244 (1992).
Gurrieri, F. et al. Physical mapping of the holoprosencephaly critical region on chromosome 7q36. Nature Genet. 3, 247–251 (1993).
Muenke, M. et al. Linkage of a human brain malformation, familial holoprosencephaly, to chromosome 7 and evidence for genetic heterogeneity. Proc. natn. Acad. Sci. U.S.A. 91, 8102–8106 (1994).
Encyclopaedia of the Mouse Genome Electronic Database. (Jackson Laboratories, Bar Harbor, 1995).
Tsukurov, O. et al. A complex bilateral polysyndactyly disease locus maps to chromosome 7q36. Nature Genet. 6, 282–286 (1994).
Heutink, P. et al. The gene for triphalangeal thumb maps to the subtelomeric region of chromosome 7q. Nature Genet. 6, 287–292 (1994).
Say, B. & Gerald, P.S. A new polydactyly/imperforate anus/vertebral anomalies syndrome? Lancet 2, 688 (1968).
Morichon-Delvallez, N., Delezoide, A.L., & Vekemans, M. Holoprosencephaly and sacral agenesis in a fetus with a terminal deletion 7q36→7qter. J. med. Genet. 30, 521–524 (1993).
Martin, A.O., Perrin, J.C.S., Muir, W.A., Ruch, E. & Schafer, I.A. An autosomal dominant midline cleft syndrome resembling familial holoprosencephaly. Clin. Genet. 12, 65–72 (1977).
Say, B. & Coldwell, J.G. Hereditary defect of the sacrum. Humangenetik 27, 231–234 (1975).
Kalter, H. Case reports of malformations associated with maternal diabetes: history and critique. Clin. Genet. 43, 174–179 (1993).
Tsui, L.-C., Donis-Keller, H. & Grzeschik, K.H. Report of the second international workshop on human chromosome 7 mapping, 1994. Cytogenet. Cell Genet. 71, 1–21 (1995).
Buetow, K.H. et al. Integrated human genome-wide maps constructed using the CEPH reference panel. Nature Genet. 6, 391–393 (1994).
Attwood, J. & Bryant, S. A computer program to make linkage analysis with LIPED and LINKAGE easier to perform and less prone to input errors. Ann. hum. Genet. 52, 259 (1988).
Cooperative Human Linkage Centre Electronic Database, Iowa University February 1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lynch, S., Bond, P., Copp, A. et al. A gene for autosomal dominant sacral agenesis maps to the holoprosencephaly region at 7q36. Nat Genet 11, 93–95 (1995). https://doi.org/10.1038/ng0995-93
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0995-93
This article is cited by
-
Currarino syndrome: a comprehensive genetic review of a rare congenital disorder
Orphanet Journal of Rare Diseases (2021)
-
Complete lung agenesis caused by complex genomic rearrangements with neo-TAD formation at the SHH locus
Human Genetics (2021)
-
Präsakrale Tumoren
coloproctology (2020)
-
Surgical management for a huge presacral teratoma and a meningocele in an adult with Currarino triad: a case report
Surgical Case Reports (2018)
-
A review of genetic factors contributing to the etiopathogenesis of anorectal malformations
Pediatric Surgery International (2018)