Abstract
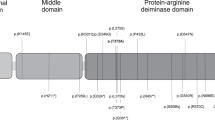
Preeclampsia is a pregnancy-associated disease with maternal symptoms but placental origin. Epigenetic inheritance is involved in some populations. By sequence analysis of 17 genes in the 10q22 region with maternal effects, we narrowed the minimal critical region linked with preeclampsia in the Netherlands to 444 kb. All but one gene in this region, which lies within a female-specific recombination hotspot, encode DNA- or RNA-binding proteins. One gene, STOX1 (also called C10orf24), contained five different missense mutations, identical between affected sisters, cosegregating with the preeclamptic phenotype and following matrilineal inheritance. Four STOX1 transcripts are expressed in early placenta, including invasive extravillus trophoblast, generating three different isoforms. All contain a winged helix domain related to the forkhead (FOX) family. The largest STOX1 isoform has exclusive nuclear or cytoplasmic expression, indicating activation and inactivation, respectively, of the PI3K-Akt-FOX pathway. Because all 38 FOX proteins and all 8 STOX1 homologs have either tyrosine or phenylalanine at position 153, the predominant Y153H variation is highly mutagenic by conservation criteria but subject to incomplete penetrance. STOX1 is a candidate for preeclampsia controlling polyploidization of extravillus trophoblast.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arngrimsson, R. et al. A genome-wide scan reveals a maternal susceptibility locus for preeclampsia on chromosome 2p13. Hum. Mol. Gen. 8, 1799–1805 (1999).
Laivuori, H. et al. Susceptibility loci for preeclampsia on chromosomes 2p25 and 9p13 in Finnish families. Am. J. Hum. Genet. 72, 168–177 (2003).
Lachmeijer, A.M. et al. A genome-wide scan for preeclampsia in the Netherlands. Eur. J. Hum. Gen. 9, 758–764 (2001).
Oudejans, C.B.M. et al. The parent-of-origin effect of 10q22 in preeclamptic females coincides with two regions clustered for genes with downregulated expression in androgenetic placentas. Mol. Hum. Reprod. 10, 589–598 (2004).
Kanayama, N. et al. Deficiency in p57kip2 expression induces preeclampsia-like symptoms in mice. Mol. Hum. Reprod. 8, 1129–1135 (2002).
Van Dijk, M. et al. Differential downregulation of αT-catenin expression in placenta: trophoblast cell type-dependent imprinting of the CTNNA3 gene. Gene Expr. Patterns 5, 61–65 (2004).
Valdez, B.C., Yang, H., Hong, E. & Sequitin, A.M. Genomic structure of newly identified paralogue of RNA helicase II/Gu: detection of pseudogenes and multiple alternatively spliced mRNAs. Gene 284, 53–61 (2002).
Deloukas, P. et al. The DNA sequence and comparative analysis of human chromosome 10. Nature 429, 375–381 (2004).
Paldi, A., Gyapay, G. & Jami, J. Imprinted chromosomal regions of the human genome display sex-specific meiotic recombination frequencies. Curr. Biol. 5, 1030–1035 (1995).
Ng, C. & Henikoff, S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 12, 436–446 (2002).
Guenther, C. & Gariga, G. Asymmetric distribution of the C. elegans HAM-1 protein in neuroblasts enables daughter cells to adopt distinct fates. Development 122, 3509–3518 (1996).
Nagase, T., Kikuno, R., Ishikawa, K.I., Hirosawa, M. & Ohara, O. Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 7, 65–73 (2000).
Stevens, K., Cirillo, L. & Zaret, K.S. Creating temperature-sensitive winged helix transcription factors. J. Biol. Chem. 275, 30471–30477 (2000).
Obsil, T., Ghirlando, R., Anderson, D.E., Burgess Hickman, A. & Dyda, F. Two 14-3-3 binding motifs are required for stable association of forkhead transcription factor FOXO4 with 14-3-3 proteins and inhibition of DNA binding. Biochemistry 42, 15264–15272 (2003).
Zhao, X. et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent- and -independent mechanisms. Biochem. J. 378, 839–849 (2004).
Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K. & Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. USA 100, 11285–11290 (2003).
Nishimura, D.Y. et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat. Genet. 19, 140–147 (1998).
Kamei, T. et al. The phosphatidylinositol 3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol. Endocrin. 16, 1469–1481 (2002).
Hattori, N., Davies, T.C., Anson-Cartwright, L. & Cross, J.C. Periodic expression of the cyclin-dependent kinase inhibitor p57(Kip2) in trophoblast giant cells defines a G2-like gap phase of the endocycle. Mol. Biol. Cell 11, 1073–1045 (2000).
Guidotti, J.E. et al. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J. Biol. Chem. 278, 19095–19101 (2003).
Harrison, G.A. et al. A genomewide linkage study of preeclampsia/eclampsia reveals evidence for a candidate region on 4q. Am. J. Hum. Genet. 60, 1158–1167 (1997).
Leegwater, P.A.J. et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat. Genet. 29, 383–388 (2001).
van Steensel, B., Delrow, J. & Henikoff, S. Chromatin profiling using targeted DNA adenine methyltransferase. Nat. Genet. 27, 304–308 (2001).
Go, A.T. et al. Detection of placental transcription factor mRNA in maternal plasma. Clin. Chem. 50, 1413–1414 (2004).
Poon, L.L., Leung, T.N., Lau, T.K., Chow, K.C. & Lo, Y.M. Differential DNA methylation between fetus and mother as a strategy for detecting fetal DNA in maternal plasma. Clin. Chem. 48, 35–41 (2002).
Riyazi, N. et al. Low-molecular-weight heparin combined with aspirin in pregnant women with trombophilia and a history of preeclampsia or fetal growth restriction: a preliminary study. Eur. J. Obstet. Gyn. Reprod. Biol. 80, 49–54 (1998).
Quenby, S., Mountfeld, S., Cartwright, J.E., Whitley, G.S. & Vince, G. Effects of low-molecular-weight and unfractionated heparin on trophoblast function. Obstet. Gynecol. 104, 354–361 (2004).
Mello, G. et al. Low-molecular weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women. Hypertension 45, 1–6 (2005).
Saleem, R.A., Banerjee-Basu, S., Berry, F.B., Baxevanis, A.D. & Walter, M.A. Analyses of the effects that disease-causing missense mutations have on the structure and function of the winged-helix protein FOXC1. Am. J. Hum. Genet. 68, 627–641 (2001).
Lewis, A. et al. Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat. Genet. 36, 1291–1295 (2004).
Acknowledgements
We thank the affected individuals, their relatives and their doctors for their support; J. Cartwright for providing SGHPL5 cells; and H. Kato for providing molar pregnancy samples. This work is supported by an Earmarked grant from the Foundation for Clinical Chemistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The VU University Medical Center has filed a patent application related to a method for diagnosing preeclampsia.
Supplementary information
Supplementary Table 1
Alignment of FOX proteins with the C10orf24 protein and its homologs. (PDF 1571 kb)
Supplementary Table 2
Missense and nonsense mutations in C10orf24 shared between preeclamptic sisters. (PDF 64 kb)
Supplementary Table 3
Primer and PCR characteristics used for coding sequence analysis of 17 genes in the 10q22 region of preeclamptic females. (PDF 41 kb)
Supplementary Table 4
Primer and PCR characteristics used for transcript analysis and cloning of C10orf24. (PDF 45 kb)
Rights and permissions
About this article
Cite this article
van Dijk, M., Mulders, J., Poutsma, A. et al. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet 37, 514–519 (2005). https://doi.org/10.1038/ng1541
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1541
This article is cited by
-
miR-30a targets STOX2 to increase cell proliferation and metastasis in hydatidiform moles via ERK, AKT, and P38 signaling pathways
Cancer Cell International (2022)
-
Alternative splicing in normal and pathological human placentas is correlated to genetic variants
Human Genetics (2021)
-
Transcriptomic analysis reveals differential gene expression, alternative splicing, and novel exons during mouse trophoblast stem cell differentiation
Stem Cell Research & Therapy (2020)
-
Pregnancy-associated cardiac dysfunction and the regulatory role of microRNAs
Biology of Sex Differences (2020)
-
Protein kinase CK2 contributes to placental development: physiological and pathological implications
Journal of Molecular Medicine (2020)