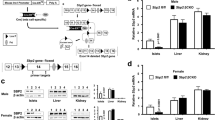
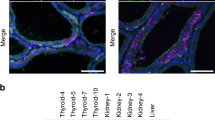
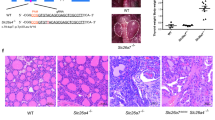
Abstract
Incorporation of selenocysteine (Sec), through recoding of the UGA stop codon, creates a unique class of proteins. Mice lacking tRNASec die in utero1, but the in vivo role of other components involved in selenoprotein synthesis is unknown, and Sec incorporation defects have not been described in humans. Deiodinases (DIOs) are selenoproteins involved in thyroid hormone metabolism. We identified three of seven siblings with clinical evidence of abnormal thyroid hormone metabolism. Their fibroblasts showed decreased DIO2 enzymatic activity not linked to the DIO2 locus. Systematic linkage analysis of genes involved in DIO2 synthesis and degradation led to the identification of an inherited Sec incorporation defect, caused by a homozygous missense mutation in SECISBP2 (also called SBP2). An unrelated child with a similar phenotype was compound heterozygous with respect to mutations in SECISBP2. Because SBP2 is epistatic to selenoprotein synthesis, these defects had a generalized effect on selenoproteins. Incomplete loss of SBP2 function probably causes the mild phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bosl, M.R., Takaku, K., Oshima, M., Nishimura, S. & Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc. Natl. Acad. Sci. USA 94, 5531–5534 (1997).
Kryukov, G.V. et al. Characterization of mammalian selenoproteomes. Science 300, 1439–1443 (2003).
Schomburg, L., Schweizer, U. & Kohrle, J. Selenium and selenoproteins in mammals: extraordinary, essential, enigmatic. Cell. Mol. Life Sci. 61, 1988–1995 (2004).
Moustafa, M.E. et al. Models for assessing the role of selenoproteins in health. J. Nutr. 133, 2494S–2496S (2003).
Schweizer, U., Schomburg, L. & Savaskan, N.E. The neurobiology of selenium: lessons from transgenic mice. J. Nutr. 134, 707–710 (2004).
Kumaraswamy, E. et al. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol. Cell. Biol. 23, 1477–1488 (2003).
Carlson, B.A. et al. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J. Biol. Chem. 279, 8011–8017 (2004).
Carlson, B.A., Xu, X.M., Gladyshev, V.N. & Hatfield, D.L. Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J. Biol. Chem. 280, 5542–5548 (2005).
Weiss Sachdev, S. & Sunde, R.A. Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and -4 in rat liver. Biochem. J. 357, 851–858 (2001).
Hatfield, D.L. & Gladyshev, V.N. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 22, 3565–3576 (2002).
Driscoll, D.M. & Copeland, P.R. Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr. 23, 17–40 (2003).
Bianco, A.C., Salvatore, D., Gereben, B., Berry, M.J. & Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 23, 38–89 (2002).
Schussler, G.C. The thyroxine-binding proteins. Thyroid 10, 141–149 (2000).
Dumitrescu, A.M., Liao, X.H., Best, T.B., Brockmann, K. & Refetoff, S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am. J. Hum. Genet. 74, 168–175 (2004).
Friesema, E.C. et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364, 1435–1437 (2004).
Dumitrescu, A. et al. On the mechanism producing the unusual thyroid phenotype in defects of the MCT8 gene. Thyroid 14, 761 (2004).
Leonard, J.L. Dibutyryl cAMP induction of type II 5′deiodinase activity in rat brain astrocytes in culture. Biochem. Biophys. Res. Commun. 151, 1164–1172 (1988).
Botero, D. et al. Ubc6p and ubc7p are required for normal and substrate-induced endoplasmic reticulum-associated degradation of the human selenoprotein type 2 iodothyronine monodeiodinase. Mol. Endocrinol. 16, 1999–2007 (2002).
Curcio-Morelli, C. et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J. Clin. Invest. 112, 189–196 (2003).
Lescure, A., Allmang, C., Yamada, K., Carbon, P. & Krol, A. cDNA cloning, expression pattern and RNA binding analysis of human selenocysteine insertion sequence (SECIS) binding protein 2. Gene 291, 279–285 (2002).
Copeland, P.R. Regulation of gene expression by stop codon recoding: selenocysteine. Gene 312, 17–25 (2003).
Schneider, M.J. et al. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 15, 2137–2148 (2001).
Olson, G.E., Winfrey, V.P., Nagdas, S.K., Hill, K.E. & Burk, R.F. Selenoprotein P is required for mouse sperm development. Biol. Reprod. 73, 201–211 (2005).
Chu, F.F. et al. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 64, 962–968 (2004).
Schussler, G.C. & Plager, J.E. Effect of preliminary purification of 131-I-thyroxine on the determination of free thyroxine in serum. J. Clin. Endocrinol. Metab. 27, 242–250 (1967).
Balzano, S. et al. Effect of total sleep deprivation on 5′-deiodinase activity of rat brown adipose tissue. Endocrinology 127, 882–890 (1990).
Schomburg, L. et al. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem. J. 370, 397–402 (2003).
Acknowledgements
We thank N.H. Scherberg and his laboratory staff for doing the tests of thyroid function in sera; all members of the families for their willingness to participate in this study; I. Abo Alnoor and A. Abomelha for their help and support; H. Hoey and M. Adress for referral of the proband of family B; M.-S. McPeek and U. Schweizer for advice; A. Hernandez and D. St. Germain for their efforts to measure DIO3 enzymatic activity in fibroblasts; J. Köhrle for his advice and help with selenoprotein analysis; K.J. Millen, G.I. Bell and D.F. Steiner for critical reading of the manuscript; F.E. Wondisford for contributing to Figure 1; and V.A. Galton for sharing with us unpublished data on the mice with combined Dio1 and Dio2 targeted disruption and for allowing us to mention these data as personal communication. This work was supported in part by grants from the US National Institutes of Health and from the Deutsche Forschungsgemeinschaft. A.M.D. is a Howard Hughes Medical Institute Predoctoral Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dumitrescu, A., Liao, XH., Abdullah, M. et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37, 1247–1252 (2005). https://doi.org/10.1038/ng1654
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1654
This article is cited by
-
The First-Ever Investigation of SNP rs119461977 in SECISBP2/SBP2 Gene and its Implications for Hypothyroidism: A Novel Case–Control Research
Indian Journal of Clinical Biochemistry (2024)
-
Gene polymorphisms and thyroid hormone signaling: implication for the treatment of hypothyroidism
Endocrine (2023)
-
Silibinin Can Promote Bone Regeneration of Selenium Hydrogel by Reducing the Oxidative Stress Pathway in Ovariectomized Rats
Calcified Tissue International (2022)
-
La resistenza e le altre sindromi da ridotta sensibilità agli ormoni tiroidei
L'Endocrinologo (2022)
-
Progress of Selenium Deficiency in the Pathogenesis of Arthropathies and Selenium Supplement for Their Treatment
Biological Trace Element Research (2022)