Abstract
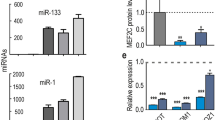
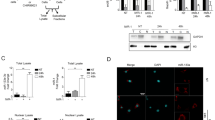
Understanding the molecular mechanisms that regulate cellular proliferation and differentiation is a central theme of developmental biology. MicroRNAs (miRNAs) are a class of regulatory RNAs of ∼22 nucleotides that post-transcriptionally regulate gene expression1,2. Increasing evidence points to the potential role of miRNAs in various biological processes3,4,5,6,7,8. Here we show that miRNA-1 (miR-1) and miRNA-133 (miR-133), which are clustered on the same chromosomal loci, are transcribed together in a tissue-specific manner during development. miR-1 and miR-133 have distinct roles in modulating skeletal muscle proliferation and differentiation in cultured myoblasts in vitro and in Xenopus laevis embryos in vivo. miR-1 promotes myogenesis by targeting histone deacetylase 4 (HDAC4), a transcriptional repressor of muscle gene expression. By contrast, miR-133 enhances myoblast proliferation by repressing serum response factor (SRF). Our results show that two mature miRNAs, derived from the same miRNA polycistron and transcribed together, can carry out distinct biological functions. Together, our studies suggest a molecular mechanism in which miRNAs participate in transcriptional circuits that control skeletal muscle gene expression and embryonic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Lee, R.C., Feinbaum, R.L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
Wightman, B., Ha, I. & Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993).
Chen, C.Z., Li, L., Lodish, H.F. & Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 303, 83–86 (2004).
He, L. et al. A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 (2005).
Giraldez, A.J. et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science 308, 833–838 (2005).
Zhao, Y., Samal, E. & Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436, 214–220 (2005).
Thomson, J.M., Parker, J., Perou, C.M. & Hammond, S.M. A custom microarray platform for analysis of microRNA gene expression. Nat. Methods 1, 47–53 (2004).
Blau, H.M. et al. Plasticity of the differentiated state. Science 230, 758–766 (1985).
Soulez, M. et al. Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol. Cell. Biol. 16, 6065–6074 (1996).
Lu, J., McKinsey, T.A., Zhang, C.L. & Olson, E.N. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell 6, 233–244 (2000).
Lee, R.C. & Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 (2001).
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 (2002).
Sempere, L.F. et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 5, R13 (2004).
Wienholds, E. et al. MicroRNA expression in zebrafish embryonic development. Science 309, 310–311 (2005).
Mansfield, J.H. et al. MicroRNA-responsive 'sensor' transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat. Genet. 36, 1079–1083 (2004).
Hutvagner, G., Simard, M.J., Mello, C.C. & Zamore, P.D. Sequence-specific inhibition of small RNA function. PLoS Biol. 2, E98 (2004).
Meister, G., Landthaler, M., Dorsett, Y. & Tuschl, T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10, 544–550 (2004).
Lewis, B.P., Shih, I.H., Jones-Rhoades, M.W., Bartel, D.P. & Burge, C.B. Prediction of mammalian microRNA targets. Cell 115, 787–798 (2003).
Kiriakidou, M. et al. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 18, 1165–1178 (2004).
Krek, A. et al. Combinatorial microRNA target predictions. Nat. Genet. 37, 495–500 (2005).
McKinsey, T.A., Zhang, C.L., Lu, J. & Olson, E.N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106–111 (2000).
Wang, D. et al. Regulation of cardiac growth and development by SRF and its cofactors. Cold Spring Harb. Symp. Quant. Biol. 67, 97–105 (2002).
Li, S. et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl. Acad. Sci. USA 102, 1082–1087 (2005).
Wang, D. et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105, 851–862 (2001).
Cao, D. et al. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol. Cell. Biol. 25, 364–376 (2005).
Kroll, K.L. & Amaya, E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173–3183 (1996).
Conlon, F.L., Sedgwick, S.G., Weston, K.M. & Smith, J.C. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122, 2427–2435 (1996).
Brown, D.D. et al. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development 132, 553–563 (2005).
Acknowledgements
We thank members of our laboratories for discussion and support; T. McKinsey for advice on the HDAC4 antibodies; E. Amaya for the dsRed construct; S. Smyth use of electroporation apparatus; M. von Drehl for histology; M. Majesky and C. Patterson for discussion and critically reading the manuscript. E.M.M. is funded by a National Science Foundation graduate research fellowship. J.M.T. is a Frederick Gardner Cottrell Postdoctoral Fellow. S.M.H. is a General Motors Cancer Research Foundation Scholar. F.L.C. was supported by the US National Institutes of Health (NIH) and the American Heart Association. D.-Z.W. is a Basil O'Connor Scholar of the March of Dimes Birth Defects Foundation and was supported by the NIH, the Muscular Dystrophy Association and an American Heart Association Grant-in-Aid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
miRNA array analysis of C2C12 cells. (PDF 692 kb)
Supplementary Fig. 2
Expression of miR-1, miR-133 and skeletal muscle differentiation marker genes in C2C12 cells. (PDF 1095 kb)
Supplementary Fig. 3
Expression of miR-1 and miR-133 in cardiac and skeletal muscle in adult mice and throughout development. (PDF 1290 kb)
Supplementary Fig. 4
Expression of miR-1 and miR-133 primary transcripts in cardiac and skeletal muscle. (PDF 145 kb)
Supplementary Fig. 5
A miR-1 and miR-133 enhancer directs reporter gene expression in cardiac and skeletal muscle. (PDF 219 kb)
Supplementary Fig. 6
Repression of a miR-133 sensor by miR-133 in C2C12 cells. (PDF 552 kb)
Supplementary Fig. 7
Sequences of the miR-1 and miR-133 target sites in the 3′ UTR of HDAC4 and SRF genes. (PDF 124 kb)
Supplementary Table 1
Sequences of oligonucleotides used in this study. (PDF 91 kb)
Rights and permissions
About this article
Cite this article
Chen, JF., Mandel, E., Thomson, J. et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38, 228–233 (2006). https://doi.org/10.1038/ng1725
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1725
This article is cited by
-
MicroRNA expression and oxidative stress markers in pectoral muscle of broiler chickens fed diets supplemented with phytobiotics composition
Scientific Reports (2024)
-
Regulation of insect behavior by non-coding RNAs
Science China Life Sciences (2024)
-
The important role of miR-1-3p in cancers
Journal of Translational Medicine (2023)
-
Construction of an RNA modification-related gene predictive model associated with prognosis and immunity in gastric cancer
BMC Bioinformatics (2023)
-
Integrated small RNA, mRNA and protein omics reveal a miRNA network orchestrating metabolic maturation of the developing human heart
BMC Genomics (2023)