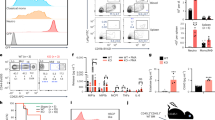
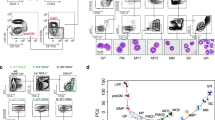
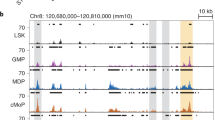
Abstract
Despite advances in the identification of lymphoid-restricted progenitor cells, the transcription factors essential for their generation remain to be identified. Here we describe an unexpected function for the myeloid oncogene product Mef2c in lymphoid development. Mef2c deficiency was associated with profound defects in the production of B cells, T cells, natural killer cells and common lymphoid progenitor cells and an enhanced myeloid output. In multipotent progenitors, Mef2c was required for the proper expression of several key lymphoid regulators and restriction of the myeloid fate. Our studies also show that Mef2c was a critical transcriptional target of the transcription factor PU.1 during lymphopoiesis. Thus, Mef2c is a crucial component of the transcriptional network that regulates cell fate 'choice' in multipotent progenitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Adolfsson, J. et al. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+c-kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15, 659–669 (2001).
Welner, R.S., Pelayo, R. & Kincade, P.W. Evolving views on the genealogy of B cells. Nat. Rev. Immunol. 8, 95–106 (2008).
Igarashi, H., Gregory, S.C., Yokota, T., Sakaguchi, N. & Kincade, P.W. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 17, 117–130 (2002).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Georgopoulos, K. et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 79, 143–156 (1994).
Scott, E.W., Simon, M.C., Anastasi, J. & Singh, H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265, 1573–1577 (1994).
Dahl, R. & Simon, M.C. The importance of PU.1 concentration in hematopoietic lineage commitment and maturation. Blood Cells Mol. Dis. 31, 229–233 (2003).
Dias, S., Xu, W., McGregor, S. & Kee, B. Transcriptional regulation of lymphocyte development. Curr. Opin. Genet. Dev. 18, 441–448 (2008).
Grounds, M.D. Towards understanding skeletal muscle regeneration. Pathol. Res. Pract. 187, 1–22 (1991).
Swanson, B.J., Jack, H.M. & Lyons, G.E. Characterization of myocyte enhancer factor 2 (MEF2) expression in B and T cells: MEF2C is a B cell-restricted transcription factor in lymphocytes. Mol. Immunol. 35, 445–458 (1998).
Martin, J.F., Schwarz, J.J. & Olson, E.N. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc. Natl. Acad. Sci. USA 90, 5282–5286 (1993).
Du, Y., Spence, S.E., Jenkins, N.A. & Copeland, N.G. Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis. Blood 106, 2498–2505 (2005).
Palmqvist, L., Pineault, N., Wasslavik, C. & Humphries, R.K. Candidate genes for expansion and transformation of hematopoietic stem cells by NUP98-HOX fusion genes. PLoS ONE 2, e768 (2007).
Krivtsov, A.V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442, 818–822 (2006).
Nagel, S. et al. MEF2C is activated by multiple mechanisms in a subset of T-acute lymphoblastic leukemia cell lines. Leukemia 22, 600–607 (2008).
Dodou, E., Verzi, M.P., Anderson, J.P., Xu, S.M. & Black, B.L. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131, 3931–3942 (2004).
Vong, L.H., Ragusa, M.J. & Schwarz, J.J. Generation of conditional Mef2cloxP/loxP mice for temporal- and tissue-specific analyses. Genesis 43, 43–48 (2005).
Kuhn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Gerber, H.P. et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417, 954–958 (2002).
Lin, Q., Schwarz, J., Bucana, C. & Olson, E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276, 1404–1407 (1997).
Arnold, M.A. et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 12, 377–389 (2007).
Lin, Q. et al. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 125, 4565–4574 (1998).
Izon, D. et al. A common pathway for dendritic cell and early B cell development. J. Immunol. 167, 1387–1392 (2001).
Xu, J. et al. Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J. Biol. Chem. 281, 9152–9162 (2006).
Dakic, A. et al. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J. Exp. Med. 201, 1487–1502 (2005).
Houston, I.B., Kamath, M.B., Schweitzer, B.L., Chlon, T.M. & DeKoter, R.P. Reduction in PU.1 activity results in a block to B-cell development, abnormal myeloid proliferation, and neonatal lethality. Exp. Hematol. 35, 1056–1068 (2007).
Kamath, M.B. et al. Dose-dependent repression of T-cell and natural killer cell genes by PU.1 enforces myeloid and B-cell identity. Leukemia 22, 1214–1225 (2008).
De Val, S. et al. Mef2c is activated directly by Ets transcription factors through an evolutionarily conserved endothelial cell-specific enhancer. Dev. Biol. 275, 424–434 (2004).
DeKoter, R.P., Lee, H.J. & Singh, H.P.U. 1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity 16, 297–309 (2002).
Dias, S., Mansson, R., Gurbuxani, S., Sigvardsson, M. & Kee, B.L. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity 29, 217–227 (2008).
Molkentin, J.D., Black, B.L., Martin, J.F. & Olson, E.N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83, 1125–1136 (1995).
Schuler, A. et al. The MADS transcription factor Mef2c is a pivotal modulator of myeloid cell fate. Blood 111, 4532–4541 (2008).
Nutt, S.L., Metcalf, D., D'Amico, A., Polli, M. & Wu, L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J. Exp. Med. 201, 221–231 (2005).
Steidl, U. et al. Essential role of Jun family transcription factors in PU.1 knockdown-induced leukemic stem cells. Nat. Genet. 38, 1269–1277 (2006).
Rao, S., Karray, S., Gackstetter, E.R. & Koshland, M.E. Myocyte enhancer factor-related B-MEF2 is developmentally expressed in B cells and regulates the immunoglobulin J chain promoter. J. Biol. Chem. 273, 26123–26129 (1998).
Porse, B.T. et al. E2F repression by C/EBPα is required for adipogenesis and granulopoiesis in vivo. Cell 107, 247–258 (2001).
Zhang, P. et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBPα. Immunity 21, 853–863 (2004).
Johnnidis, J.B. et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451, 1125–1129 (2008).
Camargo, F.D., Chambers, S.M., Drew, E., McNagny, K.M. & Goodell, M.A. Hematopoietic stem cells do not engraft with absolute efficiencies. Blood 107, 501–507 (2006).
DeKoter, R.P. & Singh, H. Regulation of B lymphocyte and macrophage development by graded expression of PU*1. Science 288, 1439–1441 (2000).
Acknowledgements
We thank J. Schwartz (Albany Medical College) and Brian Black (University of California, San Francisco) for mutant mice; G. Bell and P. Thiru for microarray data analysis; members of the Jaenisch laboratory for discussions; R. Jimenez for mouse handling and technical support; and L. Lawton (Whitehead Institute for Biomedical Research) for reagents. Supported by the Whitehead Institute Fellows (F.D.C.) and the National Institutes of Health (AI052175 to R.P.D.).
Author information
Authors and Affiliations
Contributions
S.S.-S. designed and did most of the experiments and wrote the manuscript; J.D. and R.P.D. did experiments relevant to PU.1 biology and provided intellectual input; S.L.N. provided critical reagents; and F.D.C. did experiments, designed and supervised research and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, Table 1 and Supplementary Methods (PDF 924 kb)
Rights and permissions
About this article
Cite this article
Stehling-Sun, S., Dade, J., Nutt, S. et al. Regulation of lymphoid versus myeloid fate 'choice' by the transcription factor Mef2c. Nat Immunol 10, 289–296 (2009). https://doi.org/10.1038/ni.1694
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1694
This article is cited by
-
BHLHE40/41 regulate microglia and peripheral macrophage responses associated with Alzheimer’s disease and other disorders of lipid-rich tissues
Nature Communications (2024)
-
CellComm infers cellular crosstalk that drives haematopoietic stem and progenitor cell development
Nature Cell Biology (2022)
-
A small molecular compound CC1007 induces cross-lineage differentiation by inhibiting HDAC7 expression and HDAC7/MEF2C interaction in BCR-ABL1− pre-B-ALL
Cell Death & Disease (2020)
-
Biological Characteristics and Regulation of Early Megakaryocytopoiesis
Stem Cell Reviews and Reports (2019)
-
Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion
Nature Biotechnology (2019)