Abstract
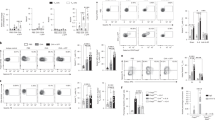
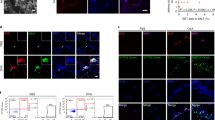
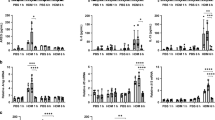
The innate immune response of airway epithelial cells to airborne allergens initiates the development of T cell responses that are central to allergic inflammation. Although proteinase allergens induce the expression of interleukin 25, we show here that epithelial matrix metalloproteinase 7 (MMP7) was expressed during asthma and was required for the maximum activity of interleukin 25 in promoting the differentiation of T helper type 2 cells. Allergen-challenged Mmp7−/− mice had less airway hyper-reactivity and production of allergic inflammatory cytokines and higher expression of retinal dehydrogenase 1. Inhibition of retinal dehydrogenase 1 restored the asthma phenotype of Mmp7−/− mice and inhibited the responses of lung regulatory T cells, whereas exogenous administration of retinoic acid attenuated the asthma phenotype. Thus, MMP7 coordinates allergic lung inflammation by activating interleukin 25 while simultaneously inhibiting retinoid-dependent development of regulatory T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kheradmand, F. et al. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169, 5904–5911 (2002).
Kiss, A. et al. A new mechanism regulating the initiation of allergic airway inflammation. J. Allergy Clin. Immunol. 120, 334–342 (2007).
Li, L. et al. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J. Immunol. 162, 2477–2487 (1999).
Zhu, J. et al. Exacerbations of bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am. J. Respir. Crit. Care Med. 164, 109–116 (2001).
Angkasekwinai, P. et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204, 1509–1517 (2007).
Moseley, T.A., Haudenschild, D.R., Rose, L. & Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Pan, G. et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J. Immunol. 167, 6559–6567 (2001).
Kim, M.R. et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood 100, 2330–2340 (2002).
Ballantyne, S.J. et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 120, 1324–1331 (2007).
Fallon, P.G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Greenlee, K.J., Werb, Z. & Kheradmand, F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 87, 69–98 (2007).
Dong, C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8, 337–348 (2008).
Parks, W.C., Wilson, C.L. & Lopez-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4, 617–629 (2004).
McMillan, S.J. et al. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J. Immunol. 172, 2586–2594 (2004).
Corry, D.B. et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 18, 995–997 (2004).
Corry, D.B. et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 3, 347–353 (2002).
Greenlee, K.J. et al. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J. Immunol. 177, 7312–7321 (2006).
Li, Q., Park, P.W., Wilson, C.L. & Parks, W.C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111, 635–646 (2002).
Wilson, C.L. et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117 (1999).
Gearing, A.J. et al. Matrix metalloproteinases and processing of pro-TNF-α. J. Leukoc. Biol. 57, 774–777 (1995).
Corry, D.B. IL-13 in allergy: home at last. Curr. Opin. Immunol. 11, 610–614 (1999).
Corry, D.B. et al. Requirements for allergen- induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 4, 344–355 (1998).
Grunig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261–2263 (1998).
Denning, T.L., Wang, Y.C., Patel, S.R., Williams, I.R. & Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 (2007).
Mucida, D. et al. Reciprocal Th-17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Connor, M.J. & Smit, M.H. Terminal-group oxidation of retinol by mouse epidermis. Inhibition in vitro and in vivo. Biochem. J. 244, 489–492 (1987).
Cheung, P.F., Wong, C.K., Ip, W.K. & Lam, C.W. IL-25 regulates the expression of adhesion molecules on eosinophils: mechanism of eosinophilia in allergic inflammation. Allergy 61, 878–885 (2006).
Fort, M.M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Zheng, T. et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Invest. 106, 1081–1093 (2000).
Swee, M., Wilson, C.L., Wang, Y., McGuire, J.K. & Parks, W.C. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J. Leukoc. Biol. 83, 1404–1412 (2008).
Liu, D. et al. Overexpression of matrix metalloproteinase-7 (MMP-7) correlates with tumor proliferation, and a poor prognosis in non-small cell lung cancer. Lung Cancer 58, 384–391 (2007).
Ito, T.K., Ishii, G., Chiba, H. & Ochiai, A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene 26, 7194–7203 (2007).
Upham, J.W. et al. Retinoic acid modulates IL-5 receptor expression and selectively inhibits eosinophil-basophil differentiation of hemopoietic progenitor cells. J. Allergy Clin. Immunol. 109, 307–313 (2002).
Takamura, K. et al. Retinoic acid inhibits interleukin-4-induced eotaxin production in a human bronchial epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L777–L785 (2004).
Takaki, H. et al. STAT6 Inhibits TGF-β1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J. Biol. Chem. 283, 14955–14962 (2008).
McGowan, S.E., Holmes, A.J. & Smith, J. Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L437–L444 (2004).
Maret, M. et al. Liposomal retinoic acids modulate asthma manifestations in mice. J. Nutr. 137, 2730–2736 (2007).
Schuster, G.U., Kenyon, N.J. & Stephensen, C.B. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J. Immunol. 180, 1834–1842 (2008).
Mora, J.R., Iwata, M. & von Andrian, U.H. Vitamin effects on the immune system: vitamins A and D take center stage. Nat. Rev. Immunol. 8, 685–698 (2008).
Coombes, J.L. & Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8, 435–446 (2008).
Sun, C.-M. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Benson, M.J., Pino-Lagos, K., Rosemblatt, M. & Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204, 1765–1774 (2007).
Elias, K.M. et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111, 1013–1020 (2008).
Peters-Golden, M. The alveolar macrophage: the forgotten cell in asthma. Am. J. Respir. Cell Mol. Biol. 31, 3–7 (2004).
Careau, E. et al. Antigen sensitization modulates alveolar macrophage functions in an asthma model. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L871–L879 (2006).
Diaz-Sanchez, D., Tsien, A., Fleming, J. & Saxon, A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J. Immunol. 158, 2406–2413 (1997).
Grumelli, S. et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 1, e8 (2004).
Montes, M., Jaensson, E.A., Orozco, A.F., Lewis, D.E. & Corry, D.B. A general method for bead-enhanced quantitation by flow cytometry. J. Immunol. Methods 317, 45–55 (2006).
Acknowledgements
We thank P. Woo Park (Children's Hospital, Harvard School of Medicine) for Mmp7−/− mice backcrossed to C57BL/6 mice; D.A. Engler, R.K. Matsunami and K. Gonzalez for technical help with proteomics analysis; N. Barrows for editorial support; and all members of the Kheradmand and Corry laboratory for comments and criticisms. Supported by the US National Institutes of Health (AI070973 and HL082487 to F.K.; AI071130 and AR050772 to C.D.; and HL075243, AI057696 and AI070973 to D.B.C.), the American Lung Association (C.D.), the Leukemia and Lymphoma Society (C.D.) and MD Anderson Cancer Center (C.D.).
Author information
Authors and Affiliations
Contributions
S.G. did animal experiments, in vitro cleavage assay, immunohistochemistry, flow cytometry, RT-PCR, ELISA and Luminex assays; P.A. did in vitro T cell experiments; W.T.B. and S.P. did airway physiology experiments; K.J.G. did proteomics analysis; A.S. made liposomal ATRA and helped with HPLC; M.S., L.S., D.R., B.S. and S.S. did the ragweed challenges of humans and ELISA of samples from allergic volunteers; P.W. obtained bronchial biopsies of asthmatic and control volunteers; S.G., F.K., D.B.C. and C.D. designed experiments; S.G. and F.K. wrote the manuscript; and P.A., D.B.C. and C.D. critically reviewed the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Tables 1–2 and Supplementary Methods (PDF 2131 kb)
Rights and permissions
About this article
Cite this article
Goswami, S., Angkasekwinai, P., Shan, M. et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol 10, 496–503 (2009). https://doi.org/10.1038/ni.1719
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1719
This article is cited by
-
Tuft cell integration of luminal states and interaction modules in tissues
Pflügers Archiv - European Journal of Physiology (2021)
-
8th international conference on management and rehabilitation of chronic respiratory failure: the long summaries – part 1
Multidisciplinary Respiratory Medicine (2015)
-
Activation of C3a receptor is required in cigarette smoke-mediated emphysema
Mucosal Immunology (2015)
-
All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma
BMC Immunology (2013)
-
Mechanisms of allergen‐specific immunotherapy
Clinical and Translational Allergy (2012)